Researchers from DTU have revealed the structure of an enzyme that can convert low-cost sugars into hard-to-produce alpha-GalNAc sugars with therapeutic properties for e.g. cancer drugs
Credit: The Novo Nordisk Foundation Center for Biosustainability
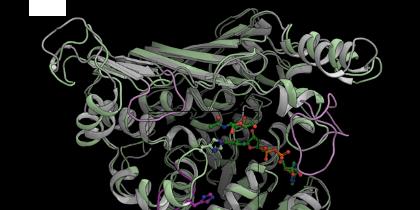
Researchers from DTU Biosustain and DTU Bioengineering have elucidated the activity of the enzyme N-acetylgalactosaminidase (GH109) whose mechanism of activity has until now been a mystery. This enzyme can convert a group of low cost, abundant so-called beta-sugars into high-value, hard-to-produce alpha-sugars with interesting therapeutic properties.
This novel work on the chemistry and catalytic activity of this enzyme opens opportunities to a wide range of applications and has now been published in the well-renowned journal ACS Catalysis.
“We are very excited to have discovered how this human symbiont uses so far unknown chemistry to feed on human sugars. Now that we understand the chemistry behind this mode of action, we can change the enzyme to make valuable sugars,” says David Teze, interim Group Leader of Enzyme Engineering at The Novo Nordisk Foundation Center for Biosustainability (DTU Biosustain) and first-author of this publication.
Bacterium with anti-cancer qualities
GH109 is found in the human gut bacterium Akkermansia muciniphila, where it degrades sugars in mucus found in the gut. When GH109 degrades the sugars of the mucus, it makes them into so-called alpha-GalNAc sugars, which it feeds on.
Biotechnologically, GH109 could allow for adding alpha-GalNAc to other compounds. This is particularly relevant, as alpha-GalNAc is the “hard to make” part of one of the most common cancer antigens, namely the Thomsen-Friedenreich antigen (Galβ1-3GalNAcα1). Thus, alpha-GalNAc sugars could potentially be formulated for cancer vaccines.
“Alpha-GalNAc containing sugars could be protective against cancer, but it is hard to synthesize enough of them for vaccines. But now, we may be able to optimize an enzyme to produce them biotechnologically,” David Teze says.
These sugars also have health-promoting features by working as potent prebiotics (food for probiotic bacteria), and other studies also suggest that they have anti-inflammatory properties. But alpha-GalNAc sugars are hard to make chemically. This is why biological production is very interesting and, hence, the function of GH109 becomes very interesting.
Using bioinformatics, mutational analysis, structural analysis, computational modeling as well as crystallography studies – the latter being conducted in one of the world’s most powerful X-ray facilities, MaxIV, in Lund, Sweden – the researchers revealed an interesting feature of GH109. The active site, which is where the enzyme activity takes place, could actually handle two types of input molecules (alpha and beta glycans). The enzyme does this by having a flexible ‘arm’ that place the right input in the active site.
Due to this revelation, the researchers have a clearer idea about how to optimise this enzyme, opening an opportunity to big scale, green production of α-GalNAc’s in the future.
###
Media Contact
Anders Østerby Mønsted
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




