Overview
Credit: COPYRIGHT (C) TOYOHASHI UNIVERSITY OF TECHNOLOGY. ALL RIGHTS RESERVED.
Overview
A research group in the doctoral program of Toyohashi University of Technology’s Department of Electrical and Electronic Information Engineering that includes a doctoral student Hirotada Gamo and specially appointed assistant professor Jin Nishida, specially appointed associate professor Atsushi Nagai, assistant professor Kazuhiro Hikima, professor Atsunori Matsuda and others, developed a large-scale manufacturing technology of Li7P3S11 solid electrolytes for all-solid-state lithium-ion secondary batteries. This method involves the addition of an excessive amount of sulfur (S) along with Li2S and P2S5, the starting materials of Li7P3S11, to a solvent containing a mixture of acetonitrile (ACN), tetrahydrofuran (THF) and a slight amount of ethanol (EtOH). This helped to shorten the reaction time from 24 hours or longer to only two minutes. The final product obtained using this method is highly pure Li7P3S11 without an impurity phase that showed high ionic conductivity of 1.2 mS cm-1 at 25 °C. These results enable us to produce a large quantity of sulfide solid electrolytes for all-solid-state batteries at low cost. The results of the research were published online by Advanced Energy and Sustainability Research on April 28, 2022.
Details
All-solid-state batteries are expected to be the next generation of batteries for electric vehicles (EVs) because they are very safe and enable a transition to high energy density and high output power. Sulfide solid electrolytes, which show good ionic conductivity and plasticity, have been actively developed with a view toward the applications for all-solid-state batteries in EVs. However, no large-scale manufacturing technology for sulfide solid electrolytes has been established at the level of commercialization, as sulfide solid electrolytes are unstable in the atmosphere and the process for synthesizing and processing them requires atmospheric control. For this reason, there is an urgent need to develop the liquid-phase manufacturing technology of sulfide solid electrolytes that offers low-cost and high scalability.
Li7P3S11 solid electrolytes exhibit high ionic conductivity and thus are one candidate solid electrolyte for all-solid-state batteries. The liquid-phase synthesis of Li7P3S11 generally occurs in an acetonitrile (ACN) reaction solvent via precursors including insoluble compounds. Conventional reaction processes like this take a long time as they go through a kinetically disadvantageous reaction from an insoluble starting material to an insoluble intermediate. Worse, it is possible that the insoluble intermediate creates non-uniformity through a complicated phase formation, leading to an increase in large-scale manufacturing costs.
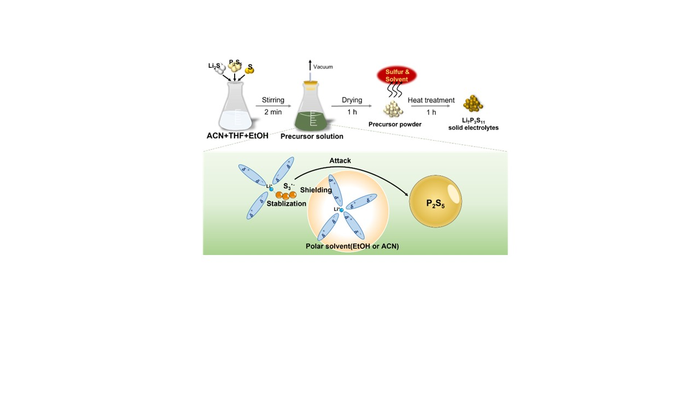
Against this background, the research group worked on the development of a technology for liquid-phase production of highly ion conductive Li7P3S11 solid electrolytes via uniform precursor solutions. It has been shown that the recently developed method can obtain a uniform precursor solution containing soluble lithium polysulfide (Li2Sx) in just two minutes, by adding Li2S and P2S5, the starting materials of Li7P3S11, and an excessive amount of S to a solvent containing a mixture of ACN, THF and a small amount of EtOH. The key to the rapid synthesis in this method is the formation of lithium polysulfide through the addition of a small amount of EtOH or an excessive amount of S.
To elucidate the mechanism of the reaction in this method, ultraviolet-visible (UV-Vis) spectroscopy was used to investigate the chemical stability of Li2Sx with and without the added EtOH. The study showed that the presence of EtOH made Li2Sx more chemically stable. Thus, the reaction in this method would take the following steps. First, lithium ions are strongly coordinated with EtOH, a highly polar solvent. Next, shielding polysulfide ions against lithium ions stabilizes highly reactive S3・– radical anions which are a kind of polysulfide. The generated S3・– attacks the P2S5, breaking the cage structure of P2S5 and causing the reaction to progress. The reaction forms lithium thiophosphate which dissolves into a highly soluble mixed solvent containing ACN and THF solvents. This may have helped to obtain uniform precursor solutions very rapidly. The final product, Li7P3S11, could be prepared in two hours without the necessity of ball milling or high energy treatment in the process of reaction.
The ion conductivity of the Li7P3S11 obtained using this method was 1.2 mS cm-1 at 25 °C, higher than the Li7P3S11 synthesized using the conventional liquid-phase synthesis method (0.8 mS cm-1) or ball milling (1.0 mS cm-1). The method proposes a new path for the synthesis of a sulfide solid electrolyte and achieves a large-scale manufacturing technology with low cost.
Future Outlook
The research team believes that the low-cost technology for the large-scale manufacturing of sulfide solid electrolytes for all-solid-state batteries proposed in this research could be important in the commercialization of EVs equipped with all-solid-state batteries. The research focused on Li7P3S11 for use as a sulfide solid electrolyte. We also want to apply this technology to the synthesis of sulfide solid electrolytes other than Li7P3S11.
This research was conducted as part of the New Energy and Industrial Technology Development Organization (NEDO)’s SOLiD-EV project (JPNP 18003) aimed at the Development of Material Evaluation Techniques for Advanced and Innovative Batteries (Phase 2).
Reference
Hirotada Gamo, Jin Nishida, Atsushi Nagai, Kazuhiro Hikima and Atsunori Matsuda, T Solution Processing via Dynamic Sulfide Radical Anions for Sulfide Solid Electrolytes, Advanced Energy and Sustainability Research, 2022, 2200019. doi.org/10.1002/aesr.202200019
Journal
Advanced Energy and Sustainability Research
DOI
10.1002/aesr.202200019
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Solution Processing via Dynamic Sulfide Radical Anions for Sulfide Solid Electrolytes
Article Publication Date
28-Apr-2022
COI Statement
The authors declare no conflict of interest.



