Credit: Toshifumi Inada, Tohoku University
The growth, death, and diseases of complex organisms rely on the flow of information — from genes in DNA, through their transcription into RNA, and then translation of that transcript into proteins, which in turn build much of the living organism.
Proteins that control this whole process are themselves subject to this overarching information flow for survival. Researchers have now discovered a previously unknown function of a group of proteins, called the Ccr4-Not complex, that may shed light on the development of diseases like Alzheimer’s.
The findings were published in Science on 17-Apr-2020.
“The Ccr4-Not complex is involved in so many aspects of gene expression that we might as well call it the ‘Swiss Army Knife’ of protein production,” says Toshifumi Inada, a professor from the Graduate School of Pharmaceutical Sciences at Tohoku University, who led the research.
The best understood aspect of Ccr4-Not’s role is its involvement in the destruction of the messenger RNA (mRNA). The mRNA molecules are like instruction manuals that tell the ribosomes, the cell’s protein-making machinery, how to construct proteins.
The amount of proteins produced by the ribosomes is crucial, and so is the speed of that production. You don’t want too many or too few, too fast or too slow. In turn, these protein levels depend on the amount of mRNA. Thus control of mRNA destruction–especially when these instruction manuals have mistakes in them–is critical in controlling protein production.
“But until now, how Ccr4-Not did this has remained elusive,” Inada adds.
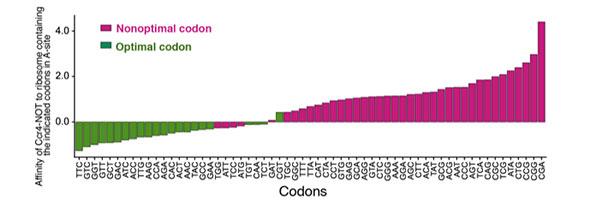
The mRNA instruction manuals are composed of multiple three-unit codes called ‘codons’ that tell the ribosomes which amino acids to use. Amino acids are the building blocks of proteins.
But even with this error-tolerant redundancy, there can still be a preference, or to use the formal term, bias, toward a particular synonym codon, because it speeds up the process. If an instruction manual for some reason doesn’t have this codon bias, the system is not working at the speed it should.
The researchers found that the Ccr4-Not complex is always on the hunt for slow-poke mRNA that don’t have this codon bias. They discovered that when the complex spots one, a part of it attaches itself to the ribosome, in turn triggering a degradation of the faulty mRNA.
But if the Ccr4-Not complex itself is faulty, missing the part that would attach to the ribosome reading the faulty mRNA, then the complex loses this crucial ability to sense and destroy these mRNA that are manufacturing proteins at the wrong speed.
Protein production that happens at the wrong speed can result in incorrect protein concentration, location or shape, all of which have been associated with a wide variety of diseases including Huntington’s and Alzheimer’s. The research offers insight into how Ccr4-Not abnormalities contribute to such illnesses via insufficient control of the rate of protein synthesis.
The researchers now want to investigate whether a normally functioning Ccr4-Not complex’s management of this rate also enables it to control protein folding and transport of proteins to their appropriate destination in the body.
###
Media Contact
Toshifumi Inada
[email protected]
Original Source
https:/
Related Journal Article
http://dx.