The secret to deciphering elderly disorders is understanding how key proteins organize themselves over time
Credit: Guilherme A. P. de Oliveira
One of the biggest difficulties in treating Parkinson’s disease, a progressive neurodegenerative disorder, is the understanding of when it starts. Now, a study published in Communications Biology by researchers at the Federal University of Rio de Janeiro, Brazil, and the University of Virginia School of Medicine, USA, may help to clarify that puzzle. For the first time, scientists observed how variants of the Parkinson’s disease-associated protein alpha-synuclein change over time and were able to identify the initial stages of protein aggregates linked to early onset of familial cases of the disease.
The characterization of these structures and their organization is fundamental to identify the early stages of the disease. It is already known that the degeneration of neurons leading to the onset of symptoms such as tremors is linked to alpha-synuclein aggregates, also called amyloid filaments, in the brain. Before forming such filaments, proteins undergo an intermediate stage, the oligomers, which are also present in the brains of Parkinson’s patients. However, there is no consensus on what mechanisms trigger aggregation, neuronal cell loss, and degeneration, neither how toxic the aggregates and the oligomers are to the cells. That is what the study tries to understand.
“A person develops Parkinson’s disease over his lifetime. The conversion from one protein stage to the other takes place slowly. The intermediate structures and the amyloid aggregates accumulate over time in the brain. So far, we don’t know which species cause the symptoms and toxicity to cells,” explains the lead author of the research Guilherme A. P. de Oliveira, researcher at the University of Virginia and professor at the UFRJ. “If we understand the protein species forming during the early stages of disease conversion, we can propose new therapies for disease detection before the symptoms appear,” he adds.
During the study, scientists compared the conversion of four variants of alpha-synuclein over time, three of them linked to early cases of the disease and the wild-type, present in cases of aging. Then, they observed significant differences in the aggregation processes of each protein and found that oligomers develop at a much greater rate in early onset cases than in aging cases of Parkinson. Such results may explain the early onset of symptoms in patients bearing these variants.
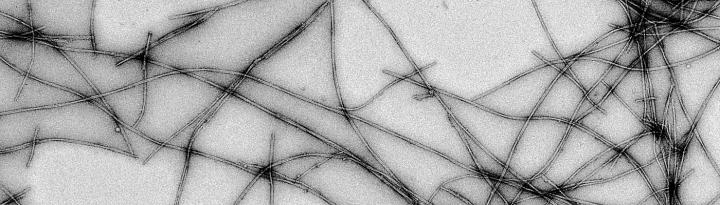
The researchers also found evidence of which protein species are important for the amyloid filaments growth. Moreover, they observed that the filaments have distinct structures depending on the protein mutation from which they originate. “Most intriguing is that not only the initial association steps are different, but also some mature filaments of hereditary cases. These filaments can twist differently depending on which mutation is present,” explains Jerson Lima Silva, second co-author and professor at UFRJ.
To perform the study, the researchers used cutting-edge bioimaging techniques. First, they used a fluorescent probe that allowed them to visualize each protein association step over time. Researchers optimized conditions in the wet lab to detect structures that were not previously shown during the course of alpha-synuclein association. Typically, the probe allows scientists to see only two stages: dark, when there is no aggregation, and light, when aggregation is present. Creating the right conditions, Oliveira and Silva managed to handle the luminosity steps and, thus, to observe the intermediate species participating on alpha-synuclein association, which would not appear in other circumstances.
The use of cryo-electron microscopy, a technique awarded with the 2017 Nobel Prize in Chemistry, was also important for the study. By allowing the visualization of biomolecules at near-atomic resolution, the scientists observed the structural organization of the amyloid filaments. According to Oliveira, the possibility of seeing such structures contributes to the development of new treatments against the disease. “By plunge freezing these samples and acquiring advanced electron microscope images, we are able to better understand these wrong protein associations in their native environment and ways to avoid their formation. I am glad that Brazil is now making part of this S&T venture,” he celebrates.
###
The paper “Alpha-synuclein stepwise aggregation reveals features of an early onset mutation in Parkinson’s disease” is published in Communications Biology.
The research was supported by the Pew Charitable Trusts to ‘de Oliveira’ as well as by the Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ), the National Council for Scientific and Technological Development (CNPq), and the National Institute of Science and Technology for Structural Biology and Bioimaging (INBEB).
Media Contact
Guilherme Guilherme A. P. de Oliveira
[email protected]
434-328-0626




