New cutting-edge technologies will accelerate discovery
Credit: Donald Danforth Plant Science Center
LOUIS, MO, September 18, 2019 – The Donald Danforth Plant Science Center has expanded the capacity of its Integrated Microscopy Facility to include high-resolution 3-D imaging at the nanoscale, single-molecule approaches, as well as automation and machine learning to enhance and accelerate research and discovery. The expansion has been led by the New Director, Kirk J. Czymmek, Ph.D., who joined the Center in June. The new name, Advanced Bioimaging Laboratory (ABL), reflects the new technologies and research capabilities. In September and October, Czymmek will be demonstrating a high-speed versatile 3D and 4D super-resolution microscope and high-pressure freezer for a state-of-the-art cryopreparation approach for light and electron microscopy, two new technologies he hopes to add as soon as funding becomes available.
“Remarkable technological advances in imaging allow us to visualize unprecedented views of the inner workings of plants as well as their interactions with other organisms and the environment. Joining the Danforth Center, with an exceptional and dedicated support team and top-notch researchers, provided the perfect setting to leverage these imaging advances to make new discoveries in plant science for the benefit of humankind,” said Czymmek.
NEW TECHNOLOGIES
ZEISS AxioZoom and Image Analysis
The new ZEISS AxioZoom microscope allows routine to advanced high-resolution multiscale fluorescence, confocal, transmitted and reflected imaging of slides, multi-well plates and intact plant specimen. This motorized system allows advanced automation to create large area maps and sophisticated multi-location and analysis routines. To support these new data acquisition capabilities we have added Intellesis for machine learning based image segmentation and ZEN Connect correlative microscopy workspace.
Array Tomography
The ABL added the DelsciMachine micromanipulator and Diatome Jumbo Histology diamond knife accessories to its Leica Ultramicrotome. These new additions allow researchers to collect long ribbons of plastic embedded “serial” sections that enable the creation of 3D images of large areas of plant cells and tissues with light and/or electron microscopy.
NEW TECHNOLOGY DEMONSTRATIONS AT THE ABL IN SEPTEMBER AND OCTOBER
ELYRA7 – September 9 – 27, 2019
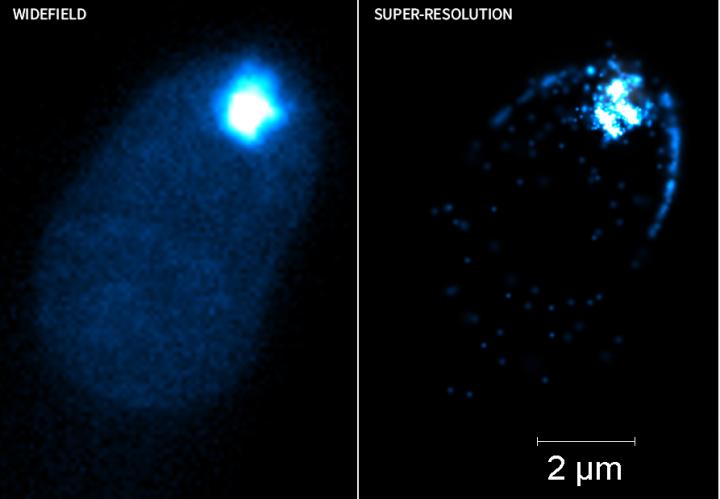
The Elyra 7 is a high-speed versatile 3D and 4D super-resolution microscope that allows researchers to break the diffraction limit using structured illumination microscopy (SIM) and single molecule localization microscopy techniques using antibodies or fluorescent proteins. The SIM approach enables a two-fold resolution increase (120nm lateral resolution) compared to traditional fluorescence microscopy by using patterned arrays when illuminating specimen. Using single molecule localization approaches, a remarkable order-of-magnitude improvement in lateral resolution can be achieved (~20nm) allowing us to see sub-cellular molecules and features with unprecedented clarity. Compare the exquisite new structural details and insights obtained from localization super-resolution microscopy of a fungal hyphal tip (Right Image) not visible in the Widefield Fluorescence image (Left Image). Coupled with incredibly high-speeds (up to 255 images/sec) and extremely high-sensitivity (85% camera detection efficiency), it is possible to see the faintest signals and the tiniest structures in living and fixed samples.
LEICA EM ICE High-Pressure Freezer – October 1 – 14, 2019
The Leica EM ICE High-Pressure Freezer is a state-of-the-art cryopreparation approach for light and electron microscopy. Using high-pressure, large specimen are physically fixed with liquid nitrogen nearly instantaneously to minimize artifacts that occur with conventional chemical fixation approaches. “Cryo-fixation is considered the gold standard in cell preservation as it freezes samples in a “life-like” state” Czymmek said. Frozen samples can be imaged under liquid nitrogen temperatures with confocal microscopy or processed for electron microscopy. The new EM ICE has easier handling and much larger formats that benefit plant structures (leaves, anthers, roots, diseased tissue) to minimize perturbation or damage from previous generation cryo-freezing systems.
Czymmek is a renowned expert in the field with over 30 years of advanced microscopy experience. He has expertise in light and electron microscopy, atomic force microscopy, single molecule imaging, super-resolution microscopy, cryo-techniques and correlative microscopy. Czymmek has over 95 refereed publications as his work has focused on developing and applying cutting-edge microscopy tools for imaging cells, tissues and biomaterials.
Prior to joining the Danforth Center, Czymmek served as Vice President of Global ZEISS Microscopy Customer Centers and oversight of eight customer centers and their teams world-wide. He joined the company in 2012 to build a world-class application, demonstration and training center for the ZEISS microscopy portfolio for North America. From 2000 to 2012 Czymmek was an Associate Professor in the Department of Biological Sciences at the University of Delaware (UD) where he worked to build an imaging capacity that led in 2001 to creation of the UD Bio-Imaging Center at the Delaware Biotechnology Institute, where he served as Director.
###
About The Donald Danforth Plant Science Center
Founded in 1998, the Donald Danforth Plant Science Center is a nonprofit research institute with a mission to improve the human condition through plant science. Research, education and outreach aim to have impact at the nexus of food security and the environment, and position the St. Louis region as a world center for plant science. The Center’s work is funded through competitive grants from many sources, including the National Institutes of Health, U.S. Department of Energy, National Science Foundation, and the Bill & Melinda Gates Foundation, and through the support of individuals and corporations. Follow us on Twitter at @DanforthCenter.
Media Contact
Karla Roeber
[email protected]
Original Source
https:/




