In a groundbreaking advancement at the crossroads of medical imaging and artificial intelligence, researchers have unveiled a novel machine learning model designed to revolutionize the preoperative differentiation of intrahepatic mass-type cholangiocarcinoma (ICC) and inflammatory pseudotumours (IPTs). These two liver conditions, despite having markedly different prognoses and treatment paths, notoriously display overlapping imaging characteristics on computed tomography (CT) scans, making accurate early diagnosis a persistent clinical challenge.
Traditional imaging modalities often fall short in distinguishing ICC, a malignant tumor arising from the bile ducts within the liver, from inflammatory pseudotumours, which are benign but can mimic cancer radiologically. This diagnostic ambiguity frequently leads to unnecessary invasive procedures, including biopsies and surgeries, subjecting patients to risks without clear benefits. Addressing this diagnostic impasse, the study spearheaded by Wang et al. leverages advanced radiomics and machine learning to enhance diagnostic precision in a clinically meaningful timeframe.
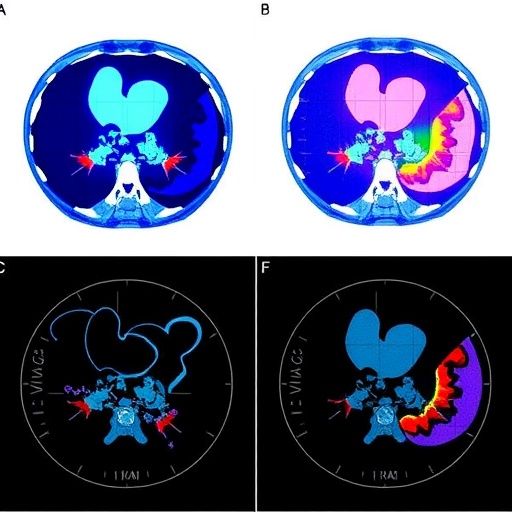
Radiomics, an innovative approach that extracts high-dimensional quantitative features from medical images, captures subtle patterns imperceptible to the human eye. By combining radiomic data derived from both plain and contrast-enhanced CT sequences with detailed clinical information, the research team developed comprehensive feature sets to train machine learning classifiers. The retrospective cohort analysis spanned nearly 16 years (May 2008 to January 2024), encompassing 146 patients confirmed by surgical and histopathological examination—112 diagnosed with ICC and 34 with hepatic IPTs—ensuring robust data fidelity for model development.
.adsslot_m0aQXJRgBk{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_m0aQXJRgBk{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_m0aQXJRgBk{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
To obtain the highest predictive accuracy, the investigators constructed fourteen distinct machine learning models for each feature subset: radiomic features alone, clinical features alone, and a hybrid set combining both radiomic and clinical data. Rigorous fivefold cross-validation coupled with exhaustive grid search optimization identified the optimal hyperparameters, ensuring that model selection accounted for potential overfitting and maintained generalizability across unseen datasets.
The results were striking. Models utilizing radiomic data from all CT sequences demonstrated impressive discriminatory power, achieving an area under the receiver operating characteristic curve (AUC) of 0.91. Integrating clinical features with comprehensive radiomic signatures further elevated performance, with the fused model reaching an outstanding AUC of 0.97, reflecting near-perfect diagnostic capability. In contrast, models relying exclusively on clinical parameters lagged behind, with an AUC of only 0.73, highlighting the superiority of imaging-derived quantitative features in this clinical context.
Delving deeper into model efficacy, the fused machine learning framework exhibited superior accuracy in recognizing ICC cases over IPTs. This asymmetry may derive from the inherently heterogeneous and complex biological behavior of cholangiocarcinomas, which manifest more distinctive radiomic patterns when compared to the inflammatory and fibrotic processes underlying pseudotumours. Such distinction is paramount clinically, as mistaking a malignant lesion for a benign counterpart can delay life-saving therapies.
The study delineates a pivotal shift towards personalized diagnostic pathways, where AI-enhanced imaging complements traditional clinical evaluation. By harnessing the latent information embedded in CT images, clinicians may soon rely less on invasive biopsies, reducing patient morbidity and healthcare costs. Moreover, this approach paves the way for future integration into routine radiological workflows, potentially enabling real-time diagnostic support during scan interpretation.
Technically, the research underscores the power of multimodal data fusion in medical prognosis. The radiomic features encompassed texture, shape, intensity, and wavelet-based parameters extracted from multiphase CT images, capturing lesion heterogeneity and microenvironmental characteristics. Combining these with clinical variables such as patient demographics and laboratory findings provided a holistic view of the tumor biology, reinforcing the machine learning algorithms’ predictive robustness.
The adoption of multiple machine learning classifiers and rigorous validation mitigated the risk of bias and enhanced model reliability. While the precise algorithms used were not detailed, the methodological rigor implied the use of state-of-the-art classifiers such as random forests, support vector machines, or gradient boosting machines, each optimized to suit the high-dimensional nature of radiomic data.
While the findings are promising, the authors acknowledge the need for prospective validation across multi-center cohorts to ensure reproducibility and account for scanner variability. Additionally, interpretability remains a challenge; deciphering which radiomic features most heavily influenced classification could shed light on the underlying biology and foster clinical trust in AI-generated insights.
In conclusion, this innovative study heralds a new era in hepatic oncology diagnostics, illustrating how machine learning models derived from CT radiomics fused with clinical data can materially improve preoperative differentiation between intrahepatic mass-type cholangiocarcinoma and inflammatory pseudotumours. As AI continues to permeate medical imaging, such efforts underscore the profound potential of computational analytics to transform patient care, fostering earlier, more accurate diagnoses and tailored treatment strategies.
The implications extend beyond liver tumors—this paradigm may be adapted to other oncological challenges characterized by diagnostic ambiguity, signaling a transformative shift towards precision medicine empowered by artificial intelligence. Continued interdisciplinary collaborations will be instrumental in translating these computational breakthroughs from research prototypes to widely accessible clinical tools.
By drastically reducing diagnostic uncertainty, this approach stands to alleviate substantial patient anxiety and optimize surgical decision-making, ultimately improving outcomes. The fusion of CT radiomics and clinical data harnessed through machine learning represents a formidable new weapon in the diagnostic arsenal against complex hepatic diseases.
As medical imaging technology advances, this study exemplifies how combining large-scale quantitative imaging features with sophisticated AI algorithms can uncover hidden diagnostic signatures that elude conventional radiological assessment. This opens avenues for non-invasive, rapid diagnostics and personalized therapeutic planning that are urgently needed in modern oncology care.
Future research building on these findings may delve into deep learning-driven feature extraction or explore integration with other imaging modalities such as MRI and PET, potentially enhancing diagnostic granularity further. Moreover, longitudinal studies assessing how model predictions correlate with patient outcomes would solidify clinical utility.
Ultimately, by embracing the convergence of radiomics and machine learning, the medical community moves closer to implementing precision diagnostics that enable truly individualized patient management strategies, marking a watershed moment for liver cancer diagnosis and beyond.
Subject of Research: Differentiating intrahepatic mass-type cholangiocarcinoma and inflammatory pseudotumours using machine learning models based on CT radiomics and clinical features.
Article Title: A machine learning model based on CT radiomics for preoperatively differentiating intrahepatic mass-type cholangiocarcinoma and inflammatory pseudotumours.
Article References:
Wang, Xc., Liang, Jh., Huang, Xy. et al. A machine learning model based on CT radiomics for preoperatively differentiating intrahepatic mass-type cholangiocarcinoma and inflammatory pseudotumours. BMC Cancer 25, 1106 (2025). https://doi.org/10.1186/s12885-025-14488-z
Image Credits: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14488-z
Tags: advanced diagnostic techniquesartificial intelligence in medical imagingclinical applications of machine learning in oncologyCT radiomics modelinflammatory pseudotumours imagingintrahepatic cholangiocarcinoma diagnosisliver tumor differentiationmachine learning in radiologypredictive modeling for liver tumorspreoperative liver tumor assessmentradiomic feature extractionreducing invasive procedures in diagnosis