In a groundbreaking study set to reshape the understanding of hepatocellular carcinoma (HCC), researchers have elucidated the role of the casein kinase 1 (CK1) family, specifically CSNK1E, in regulating crucial cellular processes such as proliferation and migration in liver cancer. This compelling insight opens new avenues for targeted therapies and enhances the understanding of molecular pathways that underpin HCC progression. As one of the most common forms of liver cancer, HCC poses significant challenges in effective treatment and management, making this research particularly relevant to cancer biology and therapeutic innovation.
Hepatocellular carcinoma has emerged as a leading cause of cancer-related mortality worldwide, particularly in regions with high rates of hepatitis B and C virus infections. As the disease advances, understanding the molecular intricacies becomes imperative for developing effective treatment modalities. The CK1 family of serine/threonine kinases plays critical roles in several cellular processes, including cell cycle regulation, signaling pathways, and gene expression. Among its members, CSNK1E has shown promise as a potential key player in cancer biology.
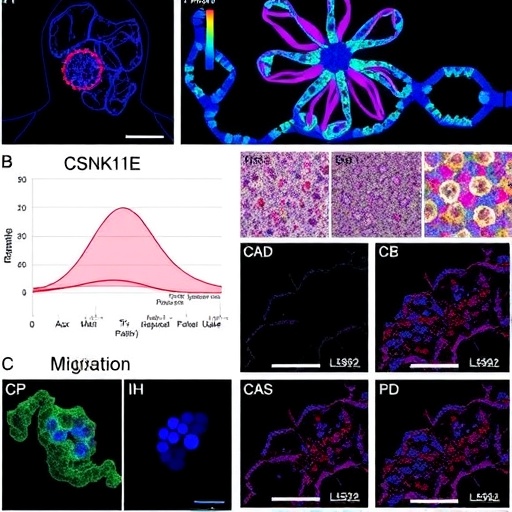
The researchers, led by Zhou et al., conducted a series of experiments to explore the function of CSNK1E in HCC cell lines and patient-derived xenografts. Their approach involved a meticulous examination of the kinase’s expression levels in hepatic tissues, revealing that CSNK1E is often upregulated in HCC compared to normal liver tissue. These findings align with previous studies that suggest aberrant kinase activity may contribute to oncogenesis. Intriguingly, when CSNK1E expression was inhibited, a marked reduction in cell proliferation and migratory capabilities was observed, underscoring the kinase’s role in facilitating tumor growth and invasion.
To dissect the underlying mechanisms through which CSNK1E exerts its effects, the team focused on downstream signaling pathways involved in tumor biology. They discovered that CSNK1E modulates several key pathways, including the Wnt/β-catenin signaling cascade, which is frequently implicated in liver cancer. By phosphorylating specific substrates involved in this pathway, CSNK1E appears to enhance β-catenin stabilization and translocation to the nucleus, a critical step for promoting the expression of target genes that drive proliferation and metastasis in HCC.
Moreover, the researchers employed various assays to assess the functional implications of CSNK1E regulation on cellular behavior. They performed colony formation assays and wound healing assays, both of which quantitatively demonstrated that silencing CSNK1E led to a significant reduction in both anchorage-independent growth and motility of HCC cells. These results collectively suggest that targeting CSNK1E may offer a novel therapeutic strategy to inhibit HCC progression by simultaneously disrupting multiple oncogenic processes.
In addition to its role in promoting proliferation and migration, CSNK1E was also found to influence the expression of several proto-oncogenes and tumor suppressor genes. The identification of these transcriptional targets reveals a more complex regulatory network orchestrated by CSNK1E, with possible implications for the development of chemoresistance. Understanding this relationship is crucial, as it may help inform the design of combination therapies that could effectively counteract resistance mechanisms that often thwart current treatment strategies.
The implications of this study extend beyond basic science; they invite translational applications that resonate within clinical oncology. By identifying CSNK1E as a potential therapeutic target, the research provides a foundation for developing small molecule inhibitors or biological agents. These agents could specifically inhibit CSNK1E activity, offering a targeted approach to mitigate the aggressive nature of HCC. This strategy aligns with the growing trend of personalized medicine, where therapies are tailored to the molecular makeup of an individual’s tumor.
Furthermore, the study raises intriguing questions about the potential role of CSNK1E in other cancers. Given the pervasive nature of CK1 family kinases in various malignancies, further exploration into the role of CSNK1E in different tumor contexts could yield significant insights. This broader perspective may lead to the identification of universal biomarkers that enhance prognostic capabilities across cancer types, potentially transforming clinical approaches to diagnosis and treatment.
The study’s findings hold particular significance as hepatocellular carcinoma continues to frustrate oncologists with its late diagnosis and poor prognostic outcomes. By targeting CSNK1E, there’s a real potential not just for improving patient survival rates but also for enhancing the quality of life for individuals battling this formidable disease. Ongoing clinical trials focused on CK1 family members will be essential in validating these findings and determining their applicability in clinical settings.
In conclusion, the work by Zhou, Wang, and Li stands at the forefront of hepatocellular carcinoma research, providing a new perspective on the molecular underpinnings of this deadly cancer. The intricate interplay between CSNK1E and various signaling pathways highlights the complexity of tumor biology and reinforces the importance of novel therapeutic strategies. As further investigations unfold, the hope is that this research will contribute to a future where precision oncology can offer targeted, effective treatments for patients suffering from hepatocellular carcinoma and other malignancies influenced by CK1 activity.
This comprehensive exploration of CSNK1E’s role in hepatocellular carcinoma illustrates the intertwining relationship between basic research and clinical application, paving the way for innovative approaches to fighting cancer. With ongoing support and interest in the cancer research community, the insights from this study have the potential to catalyze significant advancements in our overall understanding of liver cancer treatment.
Subject of Research: The role of casein kinase 1 family member CSNK1E in proliferation and migration in hepatocellular carcinoma.
Article Title: Casein kinase 1 family member CSNK1E can regulate proliferation and migration in hepatocellular carcinoma.
Article References:
Zhou, J., Wang, YH., Li, YL. et al. Casein kinase 1 family member CSNK1E can regulate proliferation and migration in hepatocellular carcinoma.
J Cancer Res Clin Oncol 151, 269 (2025). https://doi.org/10.1007/s00432-025-06321-8
Image Credits: AI Generated
DOI:
Keywords: HCC, CSNK1E, casein kinase 1, proliferation, migration, liver cancer, signaling pathways.