A groundbreaking study led by Pankaja B. Umarane and her colleagues at KLES Dr. Prabhakar Kore Hospital and MRC, in collaboration with KLE Academy of Higher Education and Research (Deemed-to-be-University), has revealed promising advancements in the early detection of prostate cancer through integrating molecular diagnostic tools. Prostate cancer remains one of the most frequently diagnosed malignancies among men globally, posing significant challenges due to its heterogeneity and diagnostic complexities. Traditional screening methods, while effective to an extent, suffer from notable drawbacks including the risk of overdiagnosis and invasive interventions that can negatively impact patient quality of life.
The research centered on using PCR-Restriction Fragment Length Polymorphism (PCR-RFLP), a molecular genetic approach, to detect mutations associated with increased prostate cancer susceptibility. PCR-RFLP leverages the amplification capabilities of polymerase chain reaction (PCR) combined with enzymatic digestion of DNA fragments, enabling the identification of polymorphisms and mutations without the necessity of high-throughput sequencing machinery. This approach offers a cost-effective and technically accessible alternative to next-generation sequencing (NGS), particularly advantageous for resource-limited settings where healthcare infrastructure is often constrained.
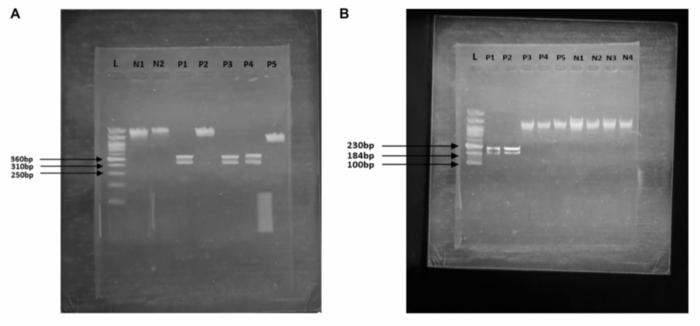
Within the study, 136 male subjects were recruited, including 66 patients with confirmed prostate cancer and 70 control individuals. The investigation targeted five genes implicated in prostate cancer risk: BRCA1, BRCA2, HOXB13, RNASEL, and ELAC2. The rationale was to discern which polymorphisms contribute most robustly to the pathogenesis of prostate cancer so that genetic markers could be established for reliable screening purposes. PCR-RFLP analysis demonstrated significant mutation frequencies in BRCA2 and HOXB13 genes, while mutations in BRCA1, RNASEL, and ELAC2 did not show statistically meaningful associations.
.adsslot_1i5yCWfsR9{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_1i5yCWfsR9{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_1i5yCWfsR9{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
BRCA2 mutations, which are well-known contributors to hereditary breast and ovarian cancers, have increasingly been recognized for their crucial role in prostate oncogenesis. The mutation rs80359550 in BRCA2 identified in this cohort was associated with more than a tenfold increase in prostate cancer risk, underscoring the gene’s pivotal involvement in DNA repair pathways that, when dysfunctional, lead to genomic instability and tumorigenesis. Similarly, HOXB13, a transcription factor essential during prostate development, harbored the mutation rs9900627, which correlated with an even higher risk factor than BRCA2. This discovery emphasizes the gene’s influence on cellular growth regulation and differentiation pathways within prostate tissue.
From a clinical perspective, these mutations provide actionable insights. By deploying PCR-RFLP genotyping in conjunction with existing diagnostic modalities such as prostate-specific antigen (PSA) levels and multiparametric magnetic resonance imaging (mpMRI), clinicians can better stratify patients based on genetic risk profiles. This stratification allows early intervention, reducing unnecessary biopsies and empowering precision treatment schemes tailored to an individual’s molecular makeup. Significantly, the affordability and scalability of PCR-RFLP ensure its adoption even in low- and middle-income countries, addressing disparities in cancer diagnostics.
Importantly, the study’s statistical analysis showcased strong genetic susceptibility linked with BRCA2 (p < 0.0001) and HOXB13 (p = 0.0139) mutations, validated by high odds ratios that establish these mutations as significant risk determinants. These robust associations highlight the potential of molecular diagnostics to complement traditional epidemiological and clinical assessments, fostering a holistic approach toward prostate cancer management. Moreover, continued research will focus on expanding the sample size and including ethnically diverse populations to affirm the universal applicability of these genetic markers.
This research further implicates familial history as a prevalent factor among affected individuals, where inherited mutations contribute considerably to prostate cancer incidence. The integration of family history data with molecular diagnostic results enhances predictive accuracy, enabling clinicians to recommend surveillance for genetically predisposed individuals. This proactive strategy is critical for reducing morbidity and mortality rates associated with advanced-stage prostate cancer by facilitating timely therapeutic interventions.
The implications for public health policy are equally profound. By endorsing low-cost, effective genetic screening tools such as PCR-RFLP for widespread clinical application, healthcare systems can optimize resource allocation and improve early diagnosis outcomes. These advancements align with the broader movement toward precision oncology, where treatment is tailored based on specific molecular characteristics rather than broad, one-size-fits-all protocols. This shift promises improved survival rates and quality of life for patients diagnosed with prostate cancer.
Furthermore, the study opens avenues for integrating molecular genetic testing into primary healthcare frameworks. Training clinicians and laboratory personnel in PCR-RFLP techniques could democratize access to genetic information, previously confined to sophisticated research laboratories. Such capacity-building initiatives are essential for empowering frontline healthcare workers to identify high-risk patients promptly and efficiently, especially in rural or underserved regions.
In conclusion, the study led by Umarane et al. serves as a critical milestone in prostate cancer diagnostics, proving that accessible molecular methods can uncover essential genetic risk factors like BRCA2 and HOXB13 mutations. The use of PCR-RFLP as a surrogate for more complex genomic sequencing heralds a new era of inclusive, affordable precision medicine, paving the way for earlier detection and personalized treatment strategies worldwide. Future investigations are anticipated to refine these findings further, ultimately integrating genetic screening into routine prostate cancer care globally.
Subject of Research: People
Article Title: Integrating molecular diagnostics for early prostate cancer detection
News Publication Date: 26-May-2025
Web References:
Journal Link: https://www.oncoscience.us/archive/v12/
DOI: http://dx.doi.org/10.18632/oncoscience.620
Image Credits: Copyright: © 2025 Umarane et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
Keywords: cancer, prostate cancer, PCR-RFLP, genetic biomarkers, molecular diagnostics, genes
Tags: advancements in cancer diagnosticschallenges in prostate cancer diagnosiscost-effective genetic testingearly detection of prostate cancermolecular diagnostic toolsnon-invasive cancer detection methodsoverdiagnosis risks in cancer screeningPCR-Restriction Fragment Length PolymorphismPCR-RFLP methodologyprostate cancer research collaborationprostate cancer susceptibility mutationsresource-limited healthcare solutions