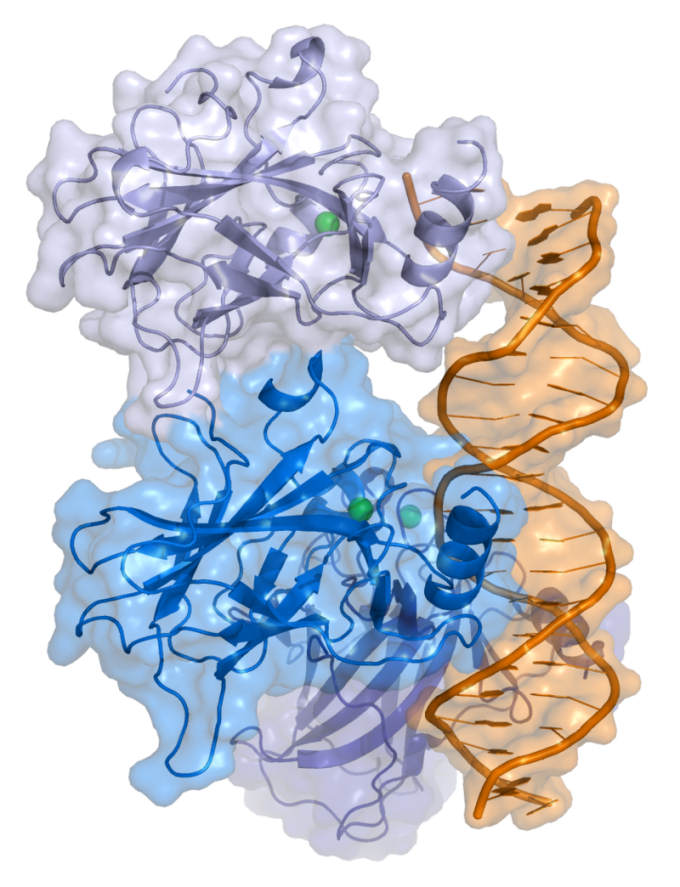
![Cartoon representation of a complex between DNA and the protein p53 (described in Cho et al. Science 265 pp. 346, 1994 [1])](https://bioengineer.org/wp-content/uploads/2016/09/800px-P53-1.jpg)
p53 has long been known to be a key protein associated with many cancers. Its main function is to suppress tumor formation in the body, and thus protect it from cancer development. However, p53 is considerably less stable compared to its two cousins, p63 and p73. Of the three proteins, p53 is the one that has deviated the most from its ancestral invertebrate version. All three proteins have a region in their sequences that is responsible for recognizing and binding to target gene sequences, called a DNA binding domain (DBD).
Loss of p53 function, which in most cases is caused by destabilizing DBD mutations, is prone to aggregation and formation of amyloid fibrils, an outcome that may be explained by its high instability. Additionally, p53 aggregates have a prion-like behavior, in which p53 mutants highjack normal p53 molecules and convert them into the inactive amyloid form.
Over 90% of p53 mutations leading to cancer development are in the DBD, making it an important target for new cancer therapies. However, the tendency of p53 to aggregate and form amyloids is an obstacle for developing new strategies.
To gain a deeper understanding of the molecular features underlying p53 DBD stability, amyloid formation, and aggregation, a research group led by Jerson Lima Silva at the Federal University of Rio de Janeiro, Brazil, used microsecond timescale molecular dynamics (MD) simulations, a computational method used for studying the precise movements of atoms over time. MD allows researchers to study biological processes at a level of detail that is difficult to obtain by conventional experiments, providing new insights into how proteins work and predicting the origins of malfunction.
In a study entitled “Aggregation tendencies in the p53 family are modulated by backbone hydrogen bonds,” published in the journal Scientific Reports, the group investigates the DBD sequence and structure of the three proteins (p53, p63, and p73) and shows that although they have similar sequences and structures in their respective DBDs, p53 is more prone to aggregation than the other two. The study shows that the innate structural weakness of p53 is explained by a high incidence of exposed backbone hydrogen bonds that are vulnerable to water attack. In contrast, p63 and p73 have better protected hydrogen bonds, and can resist water invasion and subsequent aggregation. “Our work sheds light on the molecular features underlying p53 DBD stability. The new knowledge can be used to develop strategies for stabilizing p53 and diminishing its tendency to form amyloids,” says Elio A. Cino, first author of the study.
The group is now performing studies to investigate how amyloid formation induced by common p53 mutations is associated with breast cancer, glioblastomas, and other malignant tumors, and is testing specific small molecules and peptides as a way to diminish p53 aggregation and formation of amyloid fibrils.
###
Media Contact
Jerson L. Silva
[email protected]
55-219-993-90502
http://www.publicase.com.br/
The post Computer simulation reveals p53 weak spots and opens new avenues against cancer appeared first on Scienmag.