In the relentless battle against hepatocellular carcinoma (HCC), a formidable consequence of cirrhosis, the need for precise and individualized risk stratification has never been more critical. Recent advancements in artificial intelligence (AI), radiomics, and deep learning have opened new horizons for early detection and personalized surveillance strategies. In a groundbreaking study published in the Journal of Clinical and Translational Hepatology, researchers have meticulously integrated quantitative image signatures derived from liver and spleen computed tomography (CT) scans with established clinical models. This integration promises a paradigm shift in accurately predicting HCC risk among cirrhosis patients.
Historically, predicting HCC development in cirrhotic patients has relied heavily on clinical parameters such as age, sex, albumin-bilirubin (ALBI) grade, and platelet counts. These factors are encapsulated in the age-male-ALBI-platelet (aMAP) score, a clinical model that, while valuable, has shown limitations in identifying patients at the highest risk with adequate sensitivity and specificity. Recognizing this gap, the researchers embarked on an ambitious study to enhance risk stratification by harnessing the latent information embedded in radiological imaging through advanced computational techniques.
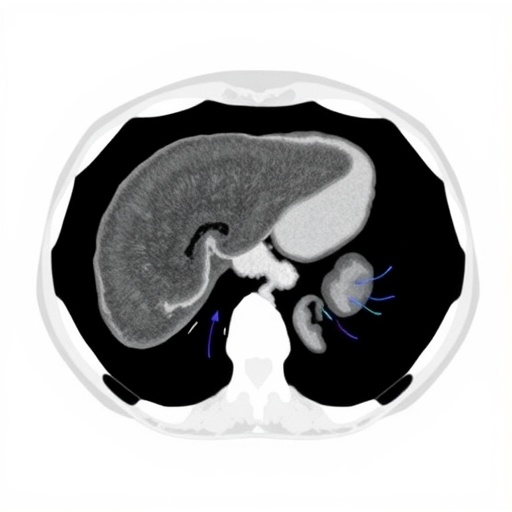
The study cohort comprised 2,411 patients enrolled over five years in a prospective, multicenter cirrhosis observational dataset in China. All participants underwent rigorous three-phase contrast-enhanced abdominal CT imaging at the point of enrollment, providing a rich repository of high-resolution liver and spleen images. These volumes were processed to extract radiomic features—a class of quantitative descriptors capturing intensity, texture, shape, and wavelet characteristics—from anatomically delineated liver and spleen regions using PyRadiomics. Complementing this approach, deep learning features were extracted through a ResNet-18 convolutional neural network architecture, fine-tuned to capture abstract representations of tissue heterogeneity and microenvironment patterns often imperceptible to the human eye.
Feature selection was a critical step given the high dimensionality of radiomic and deep learning-derived data. The investigators employed the least absolute shrinkage and selection operator (LASSO) regression technique, an advanced regularization method adept at reducing overfitting and isolating the most prognostically informative variables. This rigorous selection was essential to distill a robust set of image-derived biomarkers, enabling integration with the traditional aMAP clinical score to form the novel aMAP-CT model.
Remarkably, this composite model demonstrated superior predictive capability across multiple validation cohorts, with area under the receiver-operating characteristic curve (AUC) values ranging from 0.809 to 0.869. Such performance surpasses existing risk assessment tools, underlining the transformative potential of combining structural imaging signatures with clinical parameters. Notably, the model successfully stratified patients into distinct high-risk and low-risk groups, with the three-year cumulative incidence of HCC soaring to 26.3% within the high-risk stratum while remaining a low 1.7% in the low-risk group.
Delving deeper, the authors showcased the efficacy of a stepwise stratification approach. Initiating with the classic aMAP score to broadly categorize cirrhosis patients, the subsequent incorporation of CT-based image features refined risk categorization, pinpointing a subset of highly vulnerable individuals constituting 7% of the entire cohort. Within this nuanced subgroup, the three-year risk of HCC development was a startling 27.2%, emphasizing the precision enabled by this hybrid model. This hierarchical methodology not only streamlines patient surveillance efforts but also allocates medical resources more judiciously, focusing intensive monitoring on those most likely to benefit.
From a pathophysiological standpoint, extracting information from the spleen alongside the liver is a novel and compelling aspect of this research. The spleen often undergoes morphological and functional changes secondary to portal hypertension and systemic inflammation in cirrhosis. By incorporating spleen radiomics and deep learning signatures, the model captures an integrative view of the hepatic and extrahepatic factors contributing to tumorigenesis, thus fostering a more holistic risk assessment framework.
The use of three-phase contrast-enhanced CT imaging in this study underscores the clinical practicality of the approach. This imaging modality is widely available and routinely utilized in liver disease management, facilitating the potential rapid adoption of the aMAP-CT model in diverse healthcare settings. Furthermore, the automated nature of radiomics and deep learning feature extraction minimizes operator dependency and subjective interpretation errors, enhancing reproducibility and consistency.
In terms of clinical impact, the aMAP-CT model holds promise for revolutionizing HCC surveillance protocols. Traditional blanket screening approaches often suffer from low yield and considerable cost, not to mention the psychological and logistical burdens imposed on patients. A stratification tool of this caliber can enable personalized surveillance intervals and intensity, potentially enabling earlier tumor detection when therapeutic interventions are more effective and survival rates significantly improved.
Ethical considerations and rigorous peer review have underpinned this study’s publication in the Journal of Clinical and Translational Hepatology, ensuring adherence to robust scientific standards. The research represents a shining example of translational medicine where cutting-edge AI methodologies converge with clinical hepatology, highlighting the rapidly evolving role of computational tools in enhancing patient care.
Looking ahead, validation in other ethnic cohorts and integration with additional biomarkers such as genomics and serum molecular signatures may further refine and generalize the aMAP-CT model. Prospective trials assessing the real-world impact of AI-driven risk prediction on HCC surveillance outcomes and health economics will be essential to fully realize the potential benefits illuminated by this study.
In conclusion, this innovative study compellingly demonstrates that combining liver and spleen imaging signatures with clinical indices via AI-powered computational models vastly improves hepatocellular carcinoma risk stratification in cirrhosis patients. The aMAP-CT model heralds a new era of precision hepatology, offering a beacon of hope for earlier detection and better prognosis in a disease long shrouded in clinical uncertainty.
Subject of Research: Hepatocellular carcinoma risk stratification in cirrhosis patients through integration of radiomics and deep learning CT signatures with clinical models.
Article Title: Hepatocellular Carcinoma Risk Stratification for Cirrhosis Patients: Integrating Radiomics and Deep Learning Computed Tomography Signatures of the Liver and Spleen into a Clinical Model
News Publication Date: 1-Aug-2025
Web References:
Journal of Clinical and Translational Hepatology – https://www.xiahepublishing.com/journal/jcth
DOI – http://dx.doi.org/10.14218/JCTH.2025.00091
Image Credits: Jin-Lin Hou, Hong-Yang Wang, Rong Fan
Keywords: Hepatocellular carcinoma, Machine learning, Radiomics, Deep learning, Computed tomography, Cirrhosis, Risk stratification, AI in medicine