In a groundbreaking study poised to reshape our understanding of colorectal cancer (CRC), researchers have unveiled stark molecular differences between tumors arising in the right and left sides of the colon. This seminal work, recently published in Medical Oncology, focuses intently on the differential expression of two critical genes: CMTM3 and SSTR2. By illuminating the distinct genetic landscapes of right versus left colon tumors, the investigation sheds new light on tumor biology, potentially guiding future personalized treatment strategies and therapeutic innovations.
Colorectal cancer, a formidable health challenge worldwide, has long been recognized as a heterogeneous disease. Yet, the nuances that distinguish tumors based on their anatomical origin within the colon have remained elusive. This study dives deep into the molecular fabric that underpins these differences, offering a nuanced perspective that transcends traditional pathological classifications. The researchers utilized advanced gene expression analyses to compare the levels of CMTM3 and SSTR2 in tumor samples sourced from distinct colon segments—right and left. Their findings suggest that these genes may not just be passive markers but active players influencing tumor behavior and progression.
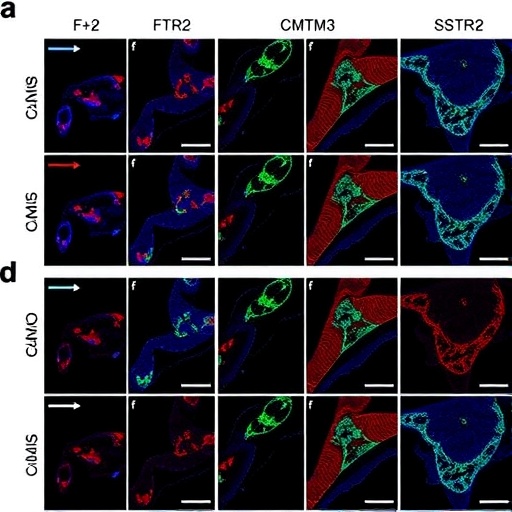
CMTM3 (CKLF-like MARVEL transmembrane domain-containing 3) emerges in this narrative as a gene with profound implications. Known to be involved in immune regulation and tumor suppression, CMTM3’s variation in expression between colon tumor locations could signal divergent tumor microenvironments and immune evasion strategies. The study reveals that alterations in CMTM3 expression are more prominent in right-sided colon tumors, raising compelling questions about how this gene modulates carcinogenesis on a molecular level within this specific colon region.
.adsslot_1kpIaDuJjn{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_1kpIaDuJjn{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_1kpIaDuJjn{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
SSTR2 (somatostatin receptor 2), another gene meticulously examined, encodes a receptor integral to regulating hormone secretion and cellular proliferation. Its expression patterns in colorectal tumors have been linked to varying responses to somatostatin analogues, compounds used in certain cancer therapies. The study reports a differential expression of SSTR2 in left-sided tumors compared to those on the right, suggesting that tumor location could influence responsiveness to receptor-targeted therapies. This discovery opens avenues to rethink therapeutic approaches, potentially tailoring them based on tumor localization.
The methodology underpinning the research reflects the rigor of contemporary molecular oncology. Tumor specimens underwent quantitative gene expression profiling using state-of-the-art molecular techniques, enabling precise measurement of mRNA levels for CMTM3 and SSTR2. These data were then meticulously correlated with clinicopathologic features, allowing the researchers to draw robust conclusions about the biological implications of their findings. The comprehensive nature of the dataset lends credibility to the notion that colorectal tumors cannot be universally characterized but must be dissected according to their anatomical and molecular idiosyncrasies.
One of the pivotal insights derived concerns the embryological and biological distinctions of the colon itself. The right and left sections derive from different embryonic origins—the midgut and hindgut, respectively—and this divergence is reflected in gene expression patterns and tumor characteristics. The study’s results support the hypothesis that such developmental origins have lasting effects on the molecular constitution of ensuing neoplasms. This emphasizes the necessity to incorporate anatomical context when considering prognostic markers and treatment regimens for CRC.
The differential gene expression of CMTM3 and SSTR2 might also intertwine with the immune landscape of colorectal tumors. Given CMTM3’s role in immune modulation, its elevated expression in right-sided tumors may contribute to a distinct immune microenvironment that affects tumor progression and patient outcomes. Similarly, varying SSTR2 levels could impact how the tumor interfaces with neuroendocrine signaling pathways, modulating growth signals in localized contexts. Together, these shifts highlight a sophisticated, location-specific interplay between genetics and tumor ecology.
From a clinical standpoint, these revelations could have transformative implications. Right-sided colon cancers are notorious for their poorer prognosis and reduced response rates to standard chemotherapy compared to left-sided cancers. Understanding the genetic underpinnings responsible for this disparity provides a molecular framework to develop location-specific biomarkers that predict therapy response with higher accuracy. This may drive a paradigm shift from a one-size-fits-all approach to precision oncology in colorectal cancer treatment.
Furthermore, the study lays foundational knowledge for exploiting these gene expression differences therapeutically. Targeting SSTR2, for instance, could be innovated upon to develop receptor-specific drugs for left-sided tumors, while reactivating or mimicking CMTM3 function might arrest right-sided tumor progression. These possibilities underscore the burgeoning field of gene-directed therapy in oncology, where genetic signatures not only diagnose but also direct treatment and monitor disease trajectory.
The significance of this study extends beyond the immediate findings to challenge prevailing clinical practice. Traditionally, CRC has been treated as a single entity, with little regard for tumor location. This research compellingly argues that the colon’s molecular ecology dictates a need for reclassification of colorectal cancer subtypes. It calls for comprehensive molecular profiling as part of routine diagnostic workflows, ensuring that treatment plans resonate with the tumor’s unique genetic identity.
Moreover, the interplay between tumor genetics and patient lifestyle or environmental factors could also be influenced by tumor location, an angle ripe for exploration. Diet, microbiota composition, and exposure to carcinogens differ along the colon, possibly driving the divergent expression patterns observed. Integrating molecular genetics with epidemiological data could further refine risk stratification and preventive strategies in colorectal cancer.
The authors advocate for expanded studies that encompass larger, multi-center cohorts, employing integrative genomic, transcriptomic, and proteomic analyses. Such holistic approaches would further clarify the complex molecular crosstalk within colonic tumors. They also emphasize the importance of longitudinal studies to understand how these gene expression disparities evolve during tumorigenesis and impact clinical outcomes over time.
Finally, this research highlights the vital role of translational science bridging the gap between bench and bedside. By decoding the molecular discrepancies of right and left colon tumors, scientists and clinicians are better equipped to innovate diagnostic, prognostic, and therapeutic tools tailored to individual cancers. As precision medicine continues to evolve, studies like these will be instrumental in transforming colorectal cancer from a monolithic disease into a spectrum of distinct, targetable entities.
In conclusion, the revelation of differential expression of CMTM3 and SSTR2 genes in right versus left colon tumors represents a significant advance in the molecular pathology of colorectal cancer. This study not only demystifies the biological heterogeneity underpinning CRC but also ignites hope for more nuanced, effective, and personalized interventions that could ultimately enhance survival and quality of life for millions affected globally.
Subject of Research: Molecular differences in gene expression (CMTM3 and SSTR2) between right and left colon tumors in colorectal cancer.
Article Title: Differences in the expression of CMTM3 and SSTR2 genes in right and left colon tumors: A molecular insight into colorectal cancer.
Article References:
Binen, T., Akbaş, E., Çolak, T., et al. Differences in the expression of CMTM3 and SSTR2 genes in right and left colon tumors: A molecular insight into colorectal cancer. Med Oncol 42, 382 (2025). https://doi.org/10.1007/s12032-025-02961-5
Image Credits: AI Generated
Tags: advanced gene expression analysis techniquesanatomical origin of colorectal tumorsCMTM3 gene expression in colorectal cancercolorectal cancer heterogeneitycolorectal cancer research advancementsgenetic landscape of colon tumorsmolecular analysis of colorectal cancerpersonalized treatment strategies for CRCright vs left colon tumor differencesSSTR2 expression in colon tumorstherapeutic innovations in cancer treatmenttumor biology and immune regulation