The process of converting DNA to proteins through an RNA is far from straightforward. Of the several types of RNA involved in the process of protein synthesis, a few may be edited mid-way. In mammals, RNA editing mostly involves converting adenosine (A) to inosine (I) through deamination, which can result in a wide range of effects. For example, A-to-I conversion can regulate gene expression in different ways and significantly alter the final synthesized protein.
Credit: Dr. Yanni Ma, Chinese Academy of Medical Sciences
The process of converting DNA to proteins through an RNA is far from straightforward. Of the several types of RNA involved in the process of protein synthesis, a few may be edited mid-way. In mammals, RNA editing mostly involves converting adenosine (A) to inosine (I) through deamination, which can result in a wide range of effects. For example, A-to-I conversion can regulate gene expression in different ways and significantly alter the final synthesized protein.
While RNA editing is an essential biological process, it is also a key underlying mechanism in some diseases, including cancer. Thus, scientists have created large-scale databases documenting RNA editing sites in various human tissues. These databases serve as useful platforms for identifying potential diagnostic or therapeutic targets from the RNA editome, which encompasses all edited RNA molecules in a given cell or tissue. Unfortunately, there are currently no databases for RNA editing in hematopoietic cells. The hematopoietic cells are unique in that they can develop into all types of blood cells including red blood cells, white blood cells, and platelets.
In a recent study published in the Chinese Medical Journal, a team of researchers led by Dr. Yanni Ma from the Chinese Academy of Medical Sciences set out to address this issue. They established a new database called ‘REDH’ that provides convenient access to the RNA editome of both healthy and malignant hematopoietic cells.
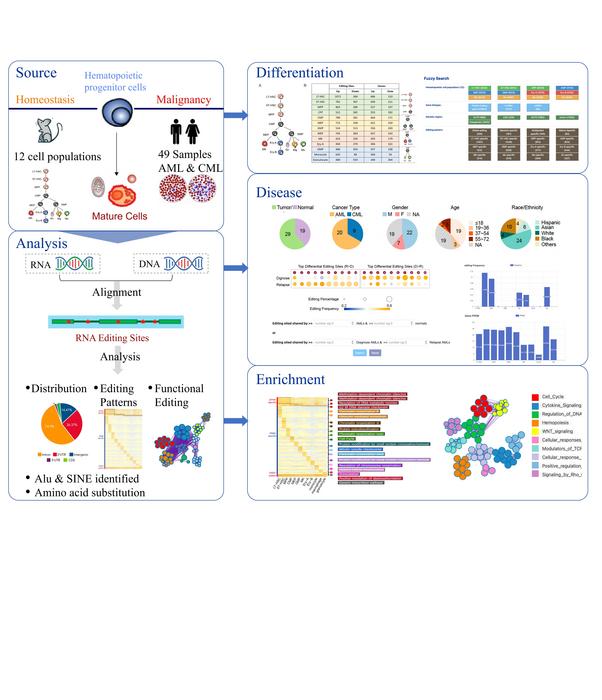
To construct the database, the researchers downloaded and analyzed RNA-sequencing (RNA-seq) data from 29 leukemia patients and 19 healthy blood donors. They also included RNA-seq data from 12 populations of mouse hematopoietic cells. Using a technique called sequence alignment, they accurately identified over 30,000 A-to-I editing sites in hematopoietic cells and nearly half a million editing events involved in hematopoietic cancers.
The database consists of four main modules: the Differentiation, Disease, Enrichment, and Knowledge modules. “Each module supports user-defined filters, dynamically generated tables based on user-defined settings, and the results are available for download,” highlights Dr. Ma. The Differentiation module contains detailed information about A-to-I editing in mouse cell populations, with the goal of assisting scientists in elucidating the correlation between RNA editing and hematopoietic development. The Disease module, on the other hand, characterizes over 400,000 A-to-I RNA editing sites from ~50 samples of two different types of leukemia. It also incorporates clinical and gene expression information from each sample, allowing for the flexible exploration of RNA editing events in individual patients or a cohort.
The Enrichment module can be used to explore the functional purpose of over 9,000 RNA editing sites. It categorizes these sites as “Chromatin organization,” “Regulation of DNA metabolic processes,” and “Regulation of chromosome organization.” This module can help researchers connect the dots between an observed trait or symptom and any associated RNA editing events. Finally, the Knowledge module contains experimentally validated RNA editing events that are known to affect hematopoiesis and hematopoietic cancers.
The REDH database will be a useful tool in guiding and accelerating research on RNA editing and its relationship with blood cell differentiation. It could lead us to a deeper understanding of blood cancers and pave the way to better treatments. The team plans to maintain and update REDH as new findings come to light, as Dr. Ma comments, “To keep the database synchronized with developments in the field of RNA editing, we will continue to monitor newly published literature and expand the types of blood diseases included in the database.” With eyes on the future, Dr. Ma concludes: “We hope our database will act as a valuable resource to explore molecular mechanisms underlying hematopoietic differentiation and identify potential therapeutic targets for leukemia and hematopoietic malignancies.”
We wish them the best of luck with REDH and echo their hopes to find insights on and treatments for blood cancers.
***
Reference
DOI: https://doi.org/10.1097/CM9.0000000000002782
Journal
Chinese Medical Journal
DOI
10.1097/CM9.0000000000002782
Method of Research
Observational study
Subject of Research
Not applicable
Article Title
REDH: A database of RNA editome in hematopoietic differentiation and malignancy
Article Publication Date
30-Jun-2023



