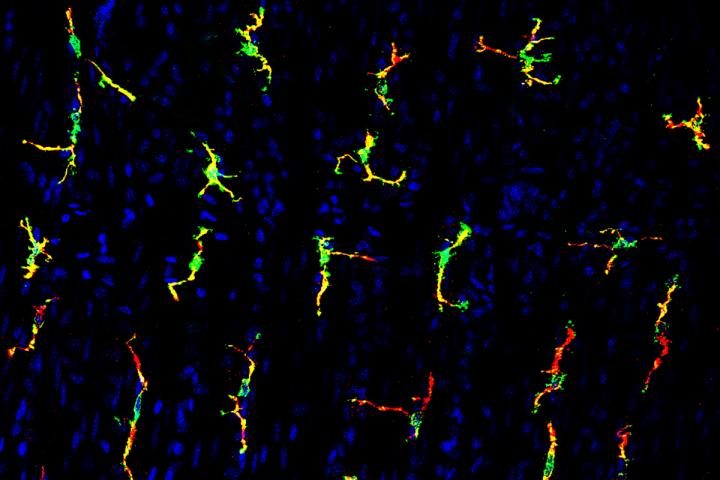
Credit: Center for the Study of Itch
Some 50 to 80 percent of cancer patients taking powerful chemotherapy drugs develop diarrhea, which can be severe and in some cases life-threatening. Their problems occur when contractions in the smooth muscle lining the gastrointestinal (GI) tract go haywire as food is digested. The same issues can occur in people with irritable bowel syndrome and inflammatory bowel disease.
For decades, scientists have thought the contraction problems originated with nerve cells in the intestines. But new research in mice at Washington University School of Medicine in St. Louis indicates that specialized immune cells called macrophages also can trigger intestinal contractions, independent of the nervous system.
The new findings, published July 17 in the journal Immunity, provide a new target to help treat chemotherapy-induced diarrhea and, potentially, diarrhea linked to other GI problems. They also raise the possibility that drugs developed to treat diarrheal-related intestinal disorders may have targeted the wrong cells, which could help explain why treatments for these conditions often aren't very effective.
"Diarrhea is a common side effect of chemotherapy that, in severe cases, can lead to death or to patients having to stop lifesaving treatment because often there are no effective therapies to control the diarrhea," said co-senior investigator Hongzhen Hu, PhD. "This research provides a new avenue to explore in developing drugs to stop such diarrhea."
Macrophages are known for their role in fighting infections, cancer and inflammation. Although the largest population of macrophages in the body resides in the gut, scientists have not understood what some of those cells were doing to help keep the GI tract healthy.
The Washington University researchers focused on a receptor on macrophages called TRPV4. These receptors are important to contractions in the gut but had been assumed to be located on nerve cells.
"We found that the macrophages themselves trigger muscle contractions in the gut without any involvement from neurons," said Hu, an associate professor of anesthesiology. "The pathway works in an entirely different way from what we had expected."
And somewhat surprisingly, the role of macrophages in intestinal motility was identified by researchers who don't normally study intestinal function. Hu and co-senior investigator Brian S. Kim, MD, are faculty members at the Washington University Center for the Study of Itch.
"People think the Itch Center exists only to solve itch-related problems, but that's an oversimplification of what we do," said Kim, co-director of the center and an assistant professor of medicine in the Division of Dermatology. "We focus on problems involving sensory perception. Itch is a great paradigm for that, but we're also concerned about things like chronic cough, migraine headaches and bowel function."
Previously, Hu and Kim had identified a role for macrophages' TRPV4 receptors in chronic itching in the skin. Here, they focused on macrophages that reside in the gut's smooth muscle layer, demonstrating that the receptors sense heat, chemical changes and the movement of food through the intestine. All of those things can trigger muscle contractions, or motility, in the gut.
In experiments involving genetically modified mice, the researchers found that animals without TRPV4 receptors on gut macrophages had poor intestinal motility. They also found that by inhibiting the actions of these receptors, they could reverse diarrhea caused by chemotherapy drugs.
"Solving problems with bowel function in chemotherapy patients is important because at least half of those patients develop diarrhea, but we'd also like to see whether these receptors on macrophages can be targeted to treat irritable bowel syndrome, which often doesn't respond well to existing drugs," Kim said.
###
Luo J, et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity (49) 1-13, July 17, 2018 (published online June 26, 2018). https://doi.org/10.1016/j.immuni.2018.04.021
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of General Medical Sciences of the National Institutes of Health (NIH). Grant numbers R01 GM 101218, R01 DK103901, P30 DK052574, K08 AR065577 and R01 AR070116.
Washington University School of Medicine's 1,300 faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children's hospitals. The School of Medicine is a leader in medical research, teaching and patient care, ranking among the top 10 medical schools in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children's hospitals, the School of Medicine is linked to BJC HealthCare.
Media Contact
Jim Dryden
[email protected]
314-286-0110
@WUSTLmed
Original Source
https://medicine.wustl.edu/?p=61479&preview=1&_ppp=911a3774a0 http://dx.doi.org/10.1016/j.immuni.2018.04.021