Covering metal catalyst surfaces with thin two-dimensional oxide materials can enhance chemical reactions
Credit: Brookhaven National Laboratory
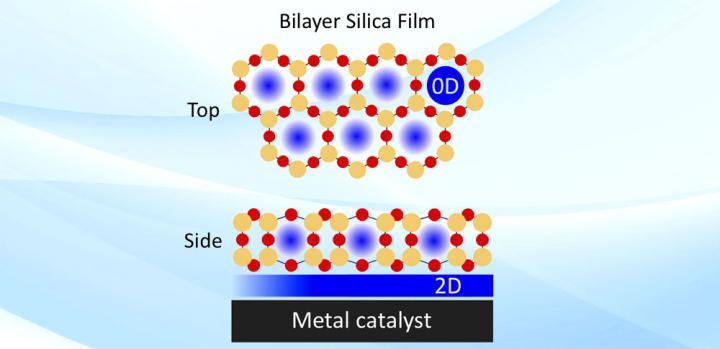
UPTON, NY–Physically confined spaces can make for more efficient chemical reactions, according to recent studies led by scientists from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory. They found that partially covering metal surfaces acting as catalysts, or materials that speed up reactions, with thin films of silica can impact the energies and rates of these reactions. The thin silica forms a two-dimensional (2-D) array of hexagonal-prism-shaped “cages” containing silicon and oxygen atoms.
“These porous silica frameworks are the thickness of only three atoms,” explained Samuel Tenney, a chemist in the Interface Science and Catalysis Group of Brookhaven Lab’s Center for Functional Nanomaterials (CFN). “If the pores were too tall, certain branches of molecules wouldn’t be able to reach the interface. There’s a particular geometry in which molecules can come in and bind, sort of like the way an enzyme and a substrate lock together. Molecules with the appropriate size can slip through the pores and interact with the catalytically active metal surface.”
“The bilayer silica is not actually anchored to the metal surface,” added Calley Eads, a research associate in the same group. “There are weak forces in between. This weak interaction allows molecules not only to penetrate the pores but also to explore the catalytic surface and find the most reactive sites and optimized reaction geometry by moving horizontally in the confined space in between the bilayer and metal. If it was anchored, the bilayer would only have one pore site for each molecule to interact with the metal.”
The scientists are discovering that the confined spaces modify different types of reactions, and they are working to understand why.
Tenney and Eads are co-corresponding authors on recently published research in Angewandte Chemie demonstrating this confinement effect for an industrially important reaction: carbon monoxide oxidation. Carbon monoxide is a toxic component of engine exhaust from vehicles and thus must be removed. With the help of an appropriate precious metal catalyst such as palladium, platinum, or rhodium, catalytic converters in vehicles combine carbon monoxide with oxygen to form carbon dioxide.
Tenney, Eads, and colleagues at the CFN and Brookhaven’s National Synchrotron Light Source II (NSLS-II) showed that covering palladium with silica boosts the amount of carbon dioxide produced by 20 percent, as compared to the reaction on bare palladium.
To achieve this performance enhancement, the scientists first had to get a full bilayer structure across the palladium surface. To do so, they heated a calibrated amount of silicon to sublimation temperatures in a high-pressure oxygen environment. In sublimation, a solid directly transforms into a gas. As the thin film of silica was being created, they probed its structure with low-energy electron diffraction. In this technique, electrons striking a material diffract in a pattern characteristic of the material’s crystalline structure.
“We continue heating until we get highly crystalline structures with well-defined pore sizes that we can use to explore the chemistry we’re interested in,” said Eads.
Here, the team tracked reactants and products and the chemical bonding environment in the 2-D confined space during oxidation of carbon monoxide, incrementally increasing the temperature. To track this information, they simultaneously conducted ambient-pressure x-ray photoelectron spectroscopy (AP-XPS) and mass spectrometry (MS) at the NSLS-II and infrared reflection-absorption spectroscopy (IRAAS) at the CFN.
“AP-XPS tells us what elements are present, whether they’re on the surface or in the gas phase,” said Tenney. “It can also give us information about the chemical oxidation state or binding geometry of the atoms–whether a carbon is bound to one or two oxygen atoms, for example. MS helps us identify the gas-phase molecules we’re seeing evolve in our system on the basis of their weight and charge. IRRAS is a fingerprint of the type of chemical bonds present between atoms and shows the conformation and orientation of carbon monoxide molecules adsorbed on the surface.”
According to co-author Dario Stacchiola, leader of the CFN Interface Science and Catalysis Group, one of the team’s unique capabilities is the ability to use complementary surface characterization tools to analyze the same sample without exposing it to air, which could cause contamination.
“Reproducibility is often a problem in catalysis,” said Stacchiola. “But we have a setup that allows us to prepare a sample in very pristine ultrahigh-vacuum conditions and expose the same sample to industrially relevant pressures of gases.”
The experimental results showed a sharp rise in the amount of carbon dioxide above a critical temperature. Below this temperature, carbon monoxide “poisons” the surface, preventing the reaction from proceeding. However, once the temperature threshold is met, molecular oxygen begins to split into two individual oxygen atoms on the palladium surface and form a surface oxide. These oxygen atoms combine with carbon monoxide to form carbon dioxide, thereby preventing poisoning.
“The confined space is changing the energetics and kinetics of the reaction to produce more carbon dioxide,” said Eads, who led the recent implementation of this new multimodal surface analysis approach for studying nanoporous films under operational conditions.
“By applying thin films on top of a traditional catalyst that has been studied for decades, we’ve introduced a “knob” to tailor the chemistry for certain reactions,” said Tenney. “Even a one-percent improvement in catalyst efficiency can translate into economic savings in large-scale production.”
“We found that a very thin layer of an inexpensive oxide can significantly boost catalytic activity without increasing the amount of the expensive precious metal used as the catalyst,” added Stacchiola.
Previously, the team studied the dynamics of the furfuryl alcohol reaction on a palladium surface covered by bilayer silica. Furfuryl alcohol is a biomass-derived molecule that can be converted into biofuel. Compared to carbon monoxide oxidation, which only makes a single product, reactions with larger and more complex biomolecules such as furfuryl alcohol can generate many undesired byproducts. Their preliminary data showed the potential for tuning the selectivity of the furfuryl alcohol reaction with the bilayer silica cover.
“Changing catalytic activity is great–that’s what we see in the carbon monoxide oxidation study,” said Stacchiola. “The next step is to prove that we can use the oxide covers to tune the selectivity for particular reactions. We think our approach can be applied broadly in catalysis.”
Last year, other members of Stacchiola’s group–along with colleagues from the CFN Theory and Computation Group, Stony Brook University (SBU), and University of Wisconsin-Milwaukee–published a related study in ACS Catalysis, a journal of the American Chemical Society (ACS). Combining experiment and theory, they discovered why the water formation reaction catalyzed by ruthenium metal is accelerated under confinement with bilayer silica.
“Chemistry in confined spaces is quite a new area of research,” said co-corresponding author Deyu Lu, a physicist in the CFN Theory and Computation Group. “In the last decade, there have been many reports that confinement impacts the chemistry, but a mechanistic understanding on the atomic scale has been largely lacking.”
In the ACS Catalysis study, the CFN team demonstrated that confinement can change the pathway by which the reaction occurs. Water formation can proceed by two possible reaction pathways: direct hydrogenation and disproportionation. The main difference is how the first hydroxyl group–oxygen bonded to hydrogen–is made. According to calculations by Lu and first author and SBU student Mengen Wang–this reaction step costs the most energy.
In the direct pathway, hydrogen molecules dissociate on the surface into two hydrogen atoms, which combine with a chemically absorbed oxygen on the surface. These hydroxyl groups combine with another hydrogen atom to make water. For the disproportionation pathway, water–which may still initially come from the direct pathway–first needs to be stabilized on the surface. Then, water can combine with a surface oxygen to make two hydroxyl groups on the surface. These hydroxyl groups can join with two hydrogen atoms to form two water molecules. These water molecules can then make more hydroxyl groups, forming a loop in the disproportionation pathway.
In lab-based AP-XPS experiments at the CFN, the team found that the temperature needed to activate the water formation reaction was much lower when silica was covering ruthenium, as compared to the metal by itself.
“The fact that the reaction takes place at lower temperatures in confinement is partially related to its lower activation energy,” explained co-corresponding author Anibal Boscoboinik, a chemist in the CFN Interface Science and Catalysis Group. “From the AP-XPS data on surface oxygen, we can indirectly derive the energy required to activate the reaction. We see that this activation energy is much lower when silica is on top of ruthenium.”
Applying a popular computational method called density functional theory, the team used supercomputers to study the energetics of the reaction. Initially, the experimentalists hypothesized that the lowered activation energy for the rate-limiting step of the reaction (making the first hydroxyl group) was due to silica pressing down on the reaction complex. However, the calculations showed that the presence of silica didn’t change this energy significantly. Rather, it changed the reaction pathway. On the bare ruthenium surface, the direct pathway was favored; in the presence of silica, water molecules stabilized on the surface, activating the disproportionation pathway.
“Without the silica cover, the water molecules desorb, and the reaction follows the direct pathway,” said Lu. “Under the silica cover, water needs to cross several kinetic energy barriers in order to leave the surface. These kinetic barriers trap water molecules on the metal surface and activate the disproportionation pathway, enabling the hydroxyl groups to be made at a much lower energy barrier, as compared to the case without the confinement effects.”
Though water formation isn’t industrially relevant, the scientists say studying this model reaction can help them understand how to leverage the confinement effects to favor certain reaction pathways for more relevant reactions. In other words, the same fundamental principle can be applied to other systems. For example, silica could be coated onto electrodes to evoke particular pathways at liquid-solid interfaces in electrochemical cells. In that case, the reaction would be the opposite–water would be dissociated into oxygen and hydrogen, a clean fuel.
“Understanding this reaction helps us to understand the reverse reaction,” said Boscoboinik, who recently published a summary of initial studies on confinement effects with 2-D porous thin films. “If we were guided by experiment alone, we would have attributed the wrong explanation. Theory proved that our initial hypothesis was incorrect and played a key role in revealing the correct reaction mechanism at the microscopic level.”
Yet, the scientists have seen other examples where silica has a pressure-related effect. In 2019, they found that bilayer silica presses down on the noble gas xenon at the interface between bilayer silica and ruthenium, inducing stronger bonding between xenon and ruthenium.
“Different effects arise from confinement,” said Stacchiola. “It’s a very interesting, rich, and mostly unexplored area. We’re excited to keep investigating chemistry in confined spaces in the coming years.”
###
The research was partially supported by the DOE Office of Science and Integrated Mesoscale Architectures for Sustainable Catalysis, a DOE Energy Frontier Research Center. Experiments at the CFN were carried out in the Proximal Probes Facility. The computations were run at the Scientific Data and Computing Center, which is part of Brookhaven’s Computational Science Initiative, and the National Energy Research Scientific Computing Center (NERSC). The AP-XPS measurements were done at the In Situ and Operando Soft X-ray Spectroscopy (IOS) beamline at an endstation built in partnership between the CFN and NSLS-II. The CFN, NERSC, and NSLS-II are all DOE Office of Science User Facilities.
Brookhaven National Laboratory is supported by the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https:/
Follow @BrookhavenLab on Twitter or find us on Facebook.
Media Contact
Ariana Manglaviti
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




