In a groundbreaking development at the University of Bristol, chemists have unveiled a pioneering technique that fundamentally transforms the way certain complex organic molecules—key components in many pharmaceutical agents—can be assembled and controlled. Their discovery, recently published in Nature, challenges long-held conventions in synthetic chemistry, introducing a versatile new method to construct tetrasubstituted alkenes. These molecules, notoriously difficult to synthesize due to their intricate four-substituent configuration around a carbon-carbon double bond, play a pivotal role in drugs such as Tamoxifen, a frontline therapy for breast cancer.
At the heart of this discovery lies the use of boron-mediated chemistry, a less common but profoundly impactful class of reactions. Traditionally, synthetic chemists have relied heavily on organic boronic esters for assembling complex alkenes. However, these esters often lead to unstable intermediates that compromise reaction efficiency and limit structural diversity. The Bristol team circumvented these challenges by harnessing boranes, a different category of boron-containing compounds. Boranes enabled “molecular gymnastics” allowing precise and modular assembly of the alkene’s core framework with unprecedented control over molecular shape and substituent placement.
One of the most astonishing facets of this research is the ability to switch the handedness—or chirality—of these tetrasubstituted alkenes simply by modifying reaction conditions. Chirality, especially in drug molecules, dictates how they interact with biological targets; one chiral form can be therapeutic while the mirror image might be inactive or even harmful. Through computational studies carried out in conjunction with chemists at Colorado State University, the team deciphered a previously unknown mechanism where the addition of a common chemical agent flips the molecule’s spatial geometry from right-handed to left-handed configuration. This mechanistic insight opens new pathways for designing drugs with tailored biological activities.
.adsslot_8v3IDSHi1P{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_8v3IDSHi1P{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_8v3IDSHi1P{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
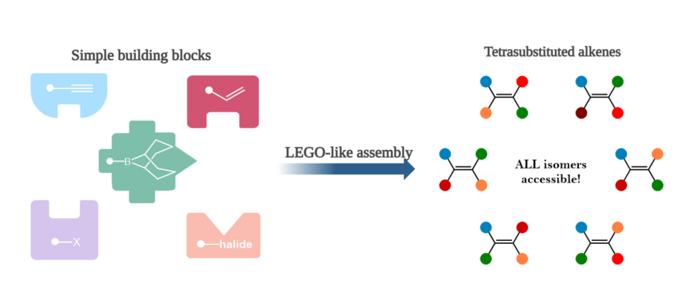
The synthetic route developed by the Bristol scientists draws an analogy to assembling complex structures from simple building blocks, akin to constructing intricate Lego models. By starting with straightforward, readily accessible molecular components, the boron-mediated process builds tetrasubstituted alkenes with high fidelity and flexibility. This modularity dramatically accelerates the synthesis of analogues, facilitating rapid exploration of molecular variations to optimize drug candidates for potency, selectivity, and reduced side effects.
Professor Varinder Aggarwal, lead author and a distinguished figure in synthetic chemistry, emphasized the transformative nature of this methodology. He noted that the ability to refine the molecular geometry of critical compounds like Tamoxifen allows for the generation of new drug variants with potentially enhanced therapeutic profiles. The implications extend beyond oncology drugs, with applications in synthesizing natural products such as γ-bisabolene, a fragrant terpene found in essential oils, demonstrating the broad utility of this chemistry for both drug discovery and materials science.
The significance of this discovery also lies in the precision and predictability that the borane-based chemistry imparts, a leap forward compared to prior methods plagued by inconsistency and limited scope. With meticulous control over which substituents are introduced and the precise spatial arrangement of these groups, chemists can now tailor molecules in ways previously deemed impractical or impossible. This capability is especially valuable in medicinal chemistry, where subtle changes in molecular shape can profoundly affect how a drug interacts with its biological target and how it is metabolized within the body.
Computational modeling provided critical insights into the reaction’s inner workings. The collaboration with researchers at Colorado State University shed light on the dynamic process by which reaction conditions influence the alkene’s stereochemistry. These simulations revealed energy landscapes and transition states that had not been appreciated before, illustrating how the boron intermediates orchestrate the assembly of complex molecules. This mechanistic understanding not only validates the experimental results but also paves the way to rationally design further reactions in this class with enhanced efficiency and specificity.
The ramifications for drug development are substantial. By leveraging this boron-mediated modular assembly, pharmaceutical chemists could efficiently generate libraries of drug candidates with diverse stereochemical and substituent profiles, identifying molecules with improved effectiveness and safety profiles at a faster pace. Given the ongoing challenges in developing cancer medicines that maintain potency while minimizing adverse effects, such advances in synthetic methodology are invaluable tools in the fight against intractable diseases.
Beyond pharmaceuticals, the approach holds promise for the creation of novel materials. The precision construction of alkenes with tailored functional groups is crucial for designing polymers, catalysts, and molecular devices with specific properties. This method’s adaptable nature suggests that it might find applications across a spectrum of chemical industries, enhancing the ability to custom-engineer molecules for targeted technological uses.
Funding for this transformative study was provided by the UK Research and Innovation (UKRI) Engineering and Physical Sciences Research Council (EPSRC), underscoring the importance of sustained support for fundamental research in synthetic chemistry. The interdisciplinary collaboration between experimentalists and computational chemists exemplifies the integrative efforts required to push boundaries in molecular science.
Looking ahead, the team envisions expanding the scope of this boron-mediated assembly to even more complex molecular architectures. By optimizing reaction parameters and exploring related boron chemistries, they aim to unlock further synthetic capabilities that will streamline the manufacture of sophisticated compounds currently inaccessible through traditional synthetic routes.
In summary, the University of Bristol’s newly reported boron-mediated modular assembly method represents a significant leap forward in the synthesis of tetrasubstituted alkenes. This breakthrough offers a versatile and controllable platform for crafting complex molecules with defined stereochemistry, promising to accelerate the development of advanced pharmaceuticals and materials. The surprising revelation that alkene geometry can be toggled by subtle changes in reaction conditions not only provides a new tool for chemists but also deepens our fundamental understanding of organic reaction mechanisms. As the scientific community builds upon these findings, the impact is poised to resonate across medicinal chemistry, natural product synthesis, and beyond.
Subject of Research: People
Article Title: ‘Boron-mediated modular assembly of tetrasubstituted alkenes’
News Publication Date: 2-Jul-2025
Web References: 10.1038/s41586-025-09209-2
Image Credits: University of Bristol
Keywords: Industrial science
Tags: advanced drug design methodsboron-mediated chemical reactionscancer drug developmentchirality in pharmaceuticalsmolecular structure controlorganic molecules assembly techniquesreducing side effects in cancer treatmentssynthetic chemistry breakthroughsTamoxifen synthesis improvementstetrasubstituted alkenes synthesisUniversity of Bristol research innovations