In the evolving landscape of oncology, patient-reported outcomes (PROs) have emerged as a vital tool to unravel the complex interplay between symptoms, functions, and quality of life. A groundbreaking study published in BMC Cancer (2025) delves deeply into this domain, harnessing advanced statistical methods and network analyses to stratify cancer patients into latent risk subgroups. The research illuminates the nuanced variations in symptom and functional networks across these groups and opens new avenues for tailored interventions that could transform patient care.
The study recruited 1,404 cancer patients from eight hospitals across two provinces in China. Employing the CA-PROM, a patient-reported outcomes measurement specifically designed for cancer patients, the researchers meticulously gathered data on health-related quality of life (HRQoL), symptom severity, and functional status. This comprehensive approach allowed for a multidimensional perspective often missing in traditional clinical assessments, which typically prioritize objective markers over subjective patient experience.
A pivotal methodological cornerstone of the study is the application of latent profile analysis (LPA). Unlike conventional subgroup classifications, LPA uses mathematical modeling to uncover hidden patterns within large datasets. The researchers used four distinct model-fit indicators to distill the patient sample into three distinct latent risk subgroups based on HRQoL: high-risk, medium-risk, and low-risk. The high-risk group constituted patients with markedly reduced quality of life, while the low-risk group exhibited relatively preserved function and fewer symptoms. This stratification underscores the heterogeneity that often confounds uniform cancer treatment approaches.
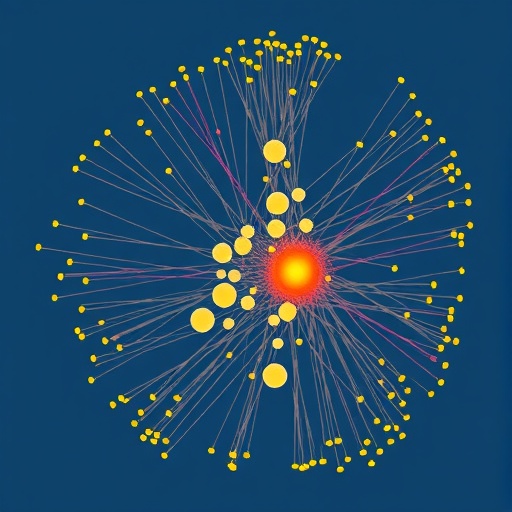
Beyond identifying subgroups, the study innovatively applied network modeling (NM) to understand how symptoms and functional impairments interconnect at an item-specific level. This approach treats symptoms and functions as nodes within a network, with “edges” representing their interactions. By calculating metrics such as expected influence (EI) and bridge EI, researchers pinpointed which symptoms or functional impairments acted as central hubs or bridges within these networks. Such nodes potentially drive the complex progression of symptom clusters affecting patients’ overall quality of life.
Importantly, the network analyses demonstrated variability in the role and influence of specific symptoms across the three risk subgroups. Symptoms like despair, gastrointestinal abnormalities, appetite loss, and social support from family and friends emerged as critical in shaping HRQoL, but their prominence differed by subgroup. For example, despair was a central symptom in the high-risk cluster, potentially exacerbating other symptoms and functional deficits, while the role of social support had a more nuanced impact across groups.
Ensuring the robustness of their findings, the researchers utilized rigorous statistical validations, including case-dropping bootstrap procedures to assess network stability and accuracy. These steps mitigated concerns surrounding sample variability and model overfitting, lending confidence to the replicability of these network structures. Furthermore, a network comparison test (NCT) revealed significant edge differences among specific symptom nodes across subgroups, emphasizing the heterogeneity in symptom interrelations depending on patients’ risk status.
One of the profound implications of this study lies in its potential to inform personalized care strategies for cancer patients. Identifying central and bridge symptoms that differ by risk subgroup offers clinicians tangible targets for intervention. Such targeted approaches could optimize symptom management, enhance functional recovery, and ultimately improve patients’ quality of life in a way that one-size-fits-all paradigms cannot.
The use of PROs as a foundation for this analysis reflects a broader shift in oncology towards incorporating patient voices directly into clinical decision-making. Traditionally, cancer management has centered on disease biomarkers and imaging studies, often neglecting the subjective burden experienced by patients. By quantifying and mapping the symptom-function landscape through PROs, this study highlights the importance of clinical assessments that prioritize patient experience alongside objective disease measures.
Moreover, the sophisticated use of network modeling in this context introduces a novel analytical lens in cancer research. It moves beyond linear cause-effect frameworks to capture the dynamic and interconnected nature of symptomatology and functional impairment. This systems-based perspective aligns with emerging theories that complex diseases like cancer involve multifaceted interactions across biological, psychological, and social domains.
Given that the study was conducted among Chinese patients, it also underscores the importance of culturally sensitive assessments in capturing accurate patient experiences. Social support, for instance, may manifest differently across cultural contexts, influencing symptom networks uniquely. Future studies might explore similar methodologies in other populations to validate and expand these insights globally.
Additionally, the differentiation of risk subgroups based on latent profiles offers practical implications for resource allocation in healthcare systems. High-risk patients, as identified in this study, may warrant closer monitoring and more intensive supportive services, while low-risk patients could benefit from routine follow-ups with a focus on maintenance therapies. Such precision in stratification enhances healthcare efficiency and prioritizes patient needs accordingly.
The interplay of despair and gastrointestinal issues as central symptoms in the high-risk group also highlights the bidirectional relationship between psychological and physical symptoms. This finding complements the growing recognition of psycho-oncology as an essential facet of cancer care, advocating for integrated mental health support to mitigate symptom burden effectively.
This study’s comprehensive approach also paves the way for leveraging artificial intelligence and machine learning in oncology. The latent profile analysis and network modeling frameworks could be enhanced by AI algorithms that predict risk subgroup transitions over time or forecast symptom emergence, further refining personalized treatment plans.
In conclusion, this transformative research bridges quantitative rigor with clinical relevance, presenting a compelling argument for integrating multidimensional patient-reported data into the fabric of cancer care. By revealing distinct latent risk subgroups and their unique symptom-function networks, the study charts a path toward interventions tailored not just to disease pathology but to the lived experiences of patients. As the oncology community moves forward, embracing such nuanced, patient-centered approaches promises to enhance quality of life—and perhaps survival—in the complex battle against cancer.
Subject of Research: Risk stratification and symptom-function networks in cancer patients based on patient-reported outcomes.
Article Title: Symptom and functional networks of patients with cancer in different latent risk subgroups based on patient-reported outcomes.
Article References:
Hu, X., Duan, Z., Li, X. et al. Symptom and functional networks of patients with cancer in different latent risk subgroups based on patient-reported outcomes. BMC Cancer 25, 872 (2025). https://doi.org/10.1186/s12885-025-14256-z
Image Credits: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14256-z
Tags: advanced statistical methods in healthcarecancer patient recruitment in clinical studiescancer patient stratification methodscancer symptom networkshealth-related quality of life in cancer patientslatent profile analysis in medical researchlatent risk groups in cancermultidimensional health data analysisnetwork analysis in cancer researchpatient-reported outcomes in oncologysubjective versus objective health assessmentstailored interventions for cancer care