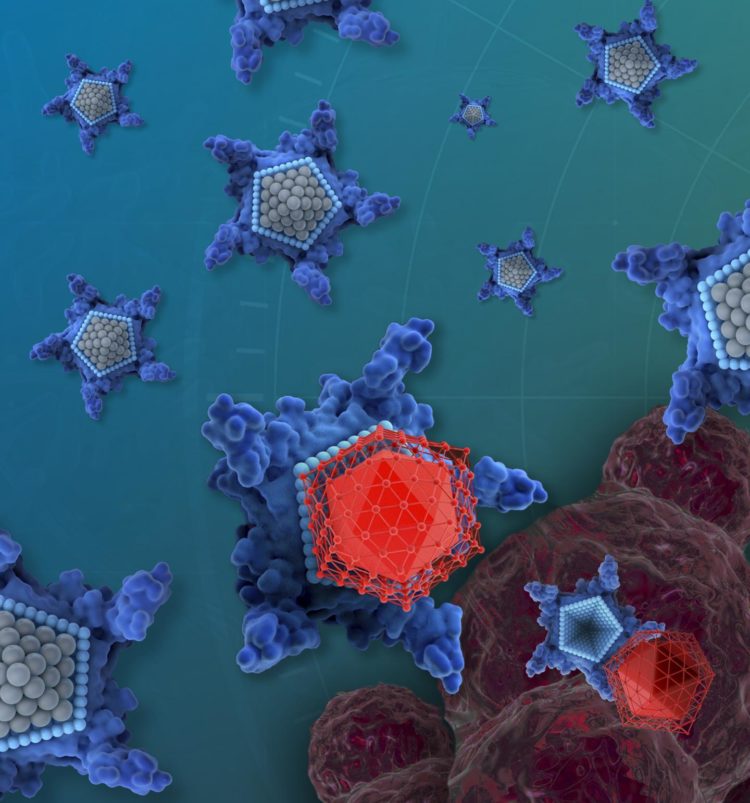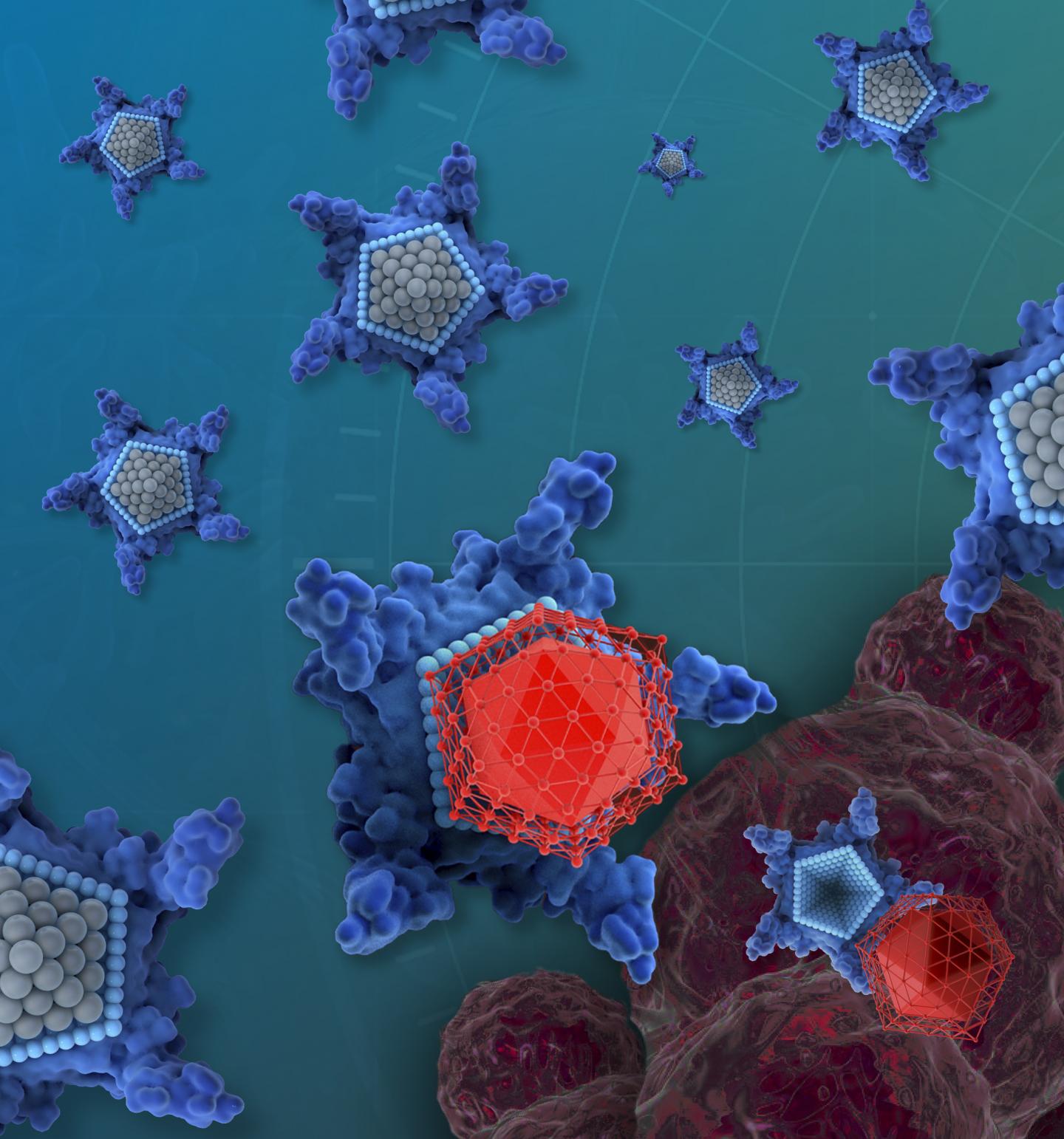
Credit: iQ Group Global
A drug candidate has been found in preclinical trials to stop tumor growth entirely, deliver more cancer-busting power than many commonly used chemotherapy drugs and do so with fewer toxic side effects and more ability to overcome resistance.
Researchers from The University of Texas at Austin, The University of Texas MD Anderson Cancer Center and Austin-based biotech firm OncoTEX report their results this week in the journal Proceedings of the National Academy of Sciences.
“As a cancer researcher and cancer survivor, I’m excited by the fact that our compound shows promise for platinum resistant ovarian cancer and platinum resistant colon cancer, both of which have poor prognoses,” said Jonathan Sessler, professor and R.P. Doherty, Jr. – Welch Regents Chair in Chemistry at UT Austin and co-principal investigator on the project.
The drug candidate, called OxaliTEX, is made of two parts: a star-shaped molecule called texaphyrin that acts like a kind of delivery truck, and a modified version of a platinum drug that acts like a toxic package for cancer cells.
Some of the most commonly used chemotherapy drugs — such as cisplatin, carboplatin and oxaliplatin — can cause toxic side effects such as kidney damage. They also often lose effectiveness as cancerous cells develop resistance. But the texaphyrin molecule is designed to be more easily absorbed by cancerous cells than healthy human cells, reducing the drug’s side effects. The new platinum drug has modifications that not only make it less toxic to healthy cells, further reducing side effects, but also that make it harder for cancerous cells to develop resistance.
The drug was developed by four inventors: Sessler; Jonathan Arambula, now vice president for research at OncoTEX; Grégory Thiabaud, a former UT Austin postdoctoral researcher, now a research associate at the Massachusetts Institute of Technology; and Zahid H. Siddik, a professor of pharmacology at MD Anderson.
The researchers compared the relative effectiveness of the new drug candidate OxaliTEX, and carboplatin, a platinum drug approved by the Food and Drug Administration commonly used to treat ovarian cancer, on mice that were carrying tumors. The mice that received carboplatin did not have a reduction in tumor growth.
Meanwhile, mice treated with OxaliTEX had 100% inhibition, meaning the tumors completely stopped growing. Two to three weeks after the drug treatment ended, tumors tended to begin growing again.
“We hope that with optimized dosing, we might be able to wipe out these tumors entirely,” Arambula said.
The researchers also compared relative toxic side effects in mice between the new drug candidate OxaliTEX and oxaliplatin, an FDA-approved platinum drug used to treat colorectal and other cancers. They found OxaliTEX had much lower toxicity.
“We created something that’s better tolerated than currently approved drugs,” Arambula said. “That’s the big message.”
Next, the researchers plan to conduct more extensive toxicology studies and, assuming those go well, hope to start a Phase 1 human clinical trial within two years.
###
The study’s other co-authors are Sajal Sen, Julie Alaniz, Ruben Munoz Macias, Greg Lyness and Alan Watts of UT Austin; Guangan He, Kathryn Shelton, Wallace Baze, Luke Segura and Rick Finch of MD Anderson; and Hyun Min Kim, Hyunseung Lee, Mi Young Cho and Kwan Soo Hong of the Korea Basic Science Institute.
A patent on the drug candidate OxaliTEX is held jointly by UT Austin and MD Anderson. The drug is licensed to the iQ Group Global and planned for further development by its subsidiary, OncoTEX. Sessler serves as a nonexecutive board member and scientific adviser for OncoTEX.
Funding for this research was provided by the Cancer Prevention and Research Institute of Texas, the U.S. National Cancer Institute and the Korea Basic Science Institute.
Media Contact
Christine Sinatra
[email protected]
512-471-4461
Related Journal Article
http://dx.