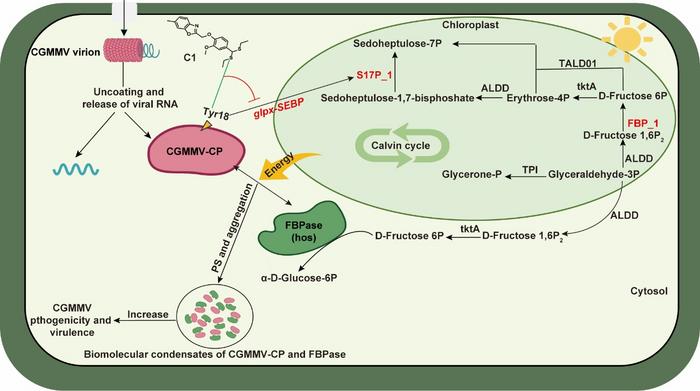
Viral replication and transmission depend heavily on the virus’s ability to hijack host cellular components. In recent years, membraneless organelles, or biomolecular condensates, have emerged as essential sites where numerous cellular processes converge. Unlike traditional membrane-bound organelles, BMCs form through LLPS, a reversible process allowing the concentration of biomolecules in dynamic compartments, thereby creating specialized microenvironments critical for diverse biological functions. CGMMV, a notorious pathogen among cucurbitaceae crops causing significant agricultural losses worldwide, adeptly commandeers this cellular mechanism by engaging host factors, primarily the enzyme fructose-1,6-bisphosphatase (FBPase), to establish replication niches.
Central to this mechanism is the interaction between the viral capsid protein (CGMMV-CP) and the host cytosolic FBPase, especially the NbFBPase isoform found in Nicotiana benthamiana, a model plant species. The capsid protein specifically binds to NbFBPase, initiating LLPS-driven formation of BMCs within host cells. This intimate interaction promotes the condensation of viral and host factors into concentrated droplet-like structures, providing a protective and efficient hub for viral genome replication and particle assembly. Experimental overexpression of NbFBPase significantly enhances CGMMV propagation, while RNA interference-mediated silencing of NbFBPase markedly restricts viral load, underscoring this host enzyme’s indispensable role.
Delving deeper into the molecular interface, the amino acid residue tyrosine at position 18 (Tyr18) within the CGMMV-CP has emerged as a pivotal determinant for viral pathogenicity. Tyr18 is instrumental not only in mediating capsid assembly but also in driving the biophysical process of LLPS, facilitating the nucleation of BMCs. Mutational analyses reveal that disruption of Tyr18 severely compromises condensate formation and subsequent virion production. This finding unravels a precise viral vulnerability — a potential “Achilles’ heel” — exploitable for antiviral intervention.
Leveraging this molecular insight, the team designed and synthesized compound C1, an innovative benzo[d]oxazol derivative, which targets Tyr18 specifically. Compound C1 exhibits superior inhibitory efficacy against CGMMV compared to established antiviral agents like Ningnanmycin, effectively interrupting the condensate formation by obstructing the crucial Tyr18-driven LLPS pathway. The compound’s mode of action demonstrates a promising antiviral strategy based on perturbing the fundamental biophysical processes essential for viral proliferation, rather than merely inhibiting enzymatic activity or viral entry.
Significantly, evidence indicates that the interaction network centered on Tyr18 and FBPase homologs is conserved across diverse cucurbitaceae species, including economically vital hosts such as cucumber and melon. This broad applicability suggests a universal viral strategy within this plant family, heightening the agricultural relevance of these findings and expanding the potential utility of compound C1 or similar analogues across multiple crops.
Beyond illuminating fundamental virology, these discoveries hold immense potential for green pesticide innovation. By targeting a defined molecular node essential for viral lifecycle progression, this research opens pathways to more sustainable, environmentally responsible disease management methods, reducing reliance on conventional broad-spectrum chemical controls. The specialized specificity of compound C1 minimizes off-target effects and environmental impact, aligning with global efforts to foster agricultural sustainability and food security.
The State Key Laboratory of Green Pesticides at Guizhou University, renowned for its expertise in eco-friendly pest control strategies, spearheaded this experimental study integrating molecular biology, biochemistry, and plant pathology. Their multidisciplinary approach exemplifies cutting-edge science aimed at addressing pressing challenges in agriculture through innovative mechanistic exploration coupled with applied chemical design.
In essence, the elucidation of CGMMV’s exploitation of host FBPase through Tyr18-mediated LLPS formation of biomolecular condensates marks a watershed moment in phytovirology. This intricate viral maneuver co-opts a host metabolic enzyme to assemble functional viral factories, a discovery that challenges traditional views of plant-virus interaction paradigms. The successful design of compound C1 ushering in effective inhibition of this process underscores the power of fundamental scientific discovery translated into practical innovation.
As future directions unfold, expanding the structural characterization of these condensates and exploring the full spectrum of CGMMV-host interactomes will further refine antiviral targeting strategies. Additionally, broad-spectrum testing of compound C1 across different cultivars and environmental conditions will validate its field applicability. Collectively, this pioneering research embodies a visionary integration of biophysical virology and chemical biology aimed at safeguarding crop health and resilience.
Subject of Research: Virus-host interaction mechanisms in plants
Article Title: (Information not provided)
News Publication Date: (Information not provided)
Web References:
http://dx.doi.org/10.1016/j.scib.2025.05.012
References:
(Information not provided)
Image Credits:
©Science China Press | Runjiang Song
Keywords:
CGMMV, biomolecular condensates, liquid-liquid phase separation, FBPase, capsid protein, Tyr18, antiviral compound C1, benzo[d]oxazol derivative, plant virology, photosynthesis modulation, cucurbitaceae viruses, green pesticides
Tags: advances in agrochemical developmentagricultural pest control innovationsantiviral strategy for plant virusesbiomolecular condensates in virus replicationCucumber Green Mottle Mosaic Viruscucurbitaceae crop diseasesFBPase enzyme and viral replicationGuizhou University research breakthroughshijacking host cellular machineryhost-virus interactions in plantsliquid-liquid phase separation in biologynovel antiviral compounds for crops