$3 million grant to Hollings Cancer Center researcher focuses on defining changes in collagen structure that are linked to disparities in breast cancer risk, progression and outcome
Credit: Medical University of South Carolina
An MUSC Hollings Cancer Center researcher received a $3 million grant from the National Cancer Institute to study how patterns of collagens can serve as biomarkers of breast cancer risk and potentially reveal clues to what might be driving health disparities.
Peggi Angel, Ph.D., is applying innovative proteomic profiling techniques to decipher the biological foundations of lethal breast cancers that impact African American women more than any other race or ethnicity. Alarmingly, African American women are more than twice as likely as white women of European descent to be diagnosed with an aggressive form of breast cancer characterized by triple negative tumor subtypes that are more likely to metastasize, seeding tumor growth in other areas of their bodies and complicating the treatments they receive.
Angel believes that collagen, one of the most abundant types of protein classes in the human body, might play a significant role in driving these disparities in breast cancer severity. Problems with collagen processing are a primary feature of triple negative breast cancer (TNBC) tumors, and these issues appear to be particularly exacerbated in breast tissues of African American women as compared to Caucasians.
In addition to the presence of wider and longer collagen fibers within breast tumors and surrounding tissues, Angel’s data indicates increased levels of a certain chemical modification that occurs in structurally important areas of collagen proteins involving hydroxylated proline, which is a major component of the protein collagen.
Hydroxyproline (HYP) modification of collagen is critical to tissue stability. The collagen “matrix” that forms the structural basis of skin, bones and other organs is a highly dynamic environment that is constantly being reorganized and renewed, she explained.
During normal processing, one collagen protein links up with two others to form a basic unit. This occurs within collagen-producing cells called fibroblasts. These units then arrange themselves outside the fibroblast, first into fibrils and then into larger fibers that form the collagen matrix. How these building blocks come together depends on patterns of chemical modifications, including HYP and others, which serve as the “glue” holding the strands together. Angel’s data suggests that ancestry-dependent HYP changes can alter this structure, making it more susceptible to rearrangements that favor tumor invasiveness.
Collagen highways
In the very early stages of breast cancer, breast tissue normally has a wavy kind of pattern of collagen. As the tumor grows, though, the normal wavy collagen fibers form into linear strands of collagen parallel to the breast tumor.
“It’s been known for many years that breast cancer fibroblasts have this very systematic way of reengineering the tissue to promote invasion. What the breast cancer fibroblasts do is start building these collagen highways at the tumor borders,” she said.
“At certain points, and this appears to happen earlier in African American women than in European American women, cancer fibroblasts at the edge of the tumor start taking over collagen production at the tumor borders. They actually start building these collagen fibers perpendicular to the tumor border extending out into nearby tissue.”
Researchers have found that this is an important way that breast cancer progresses to invasion and have linked these tissue-associated collagen signatures called TACS to breast cancer progression. They also have found that the level of those tissue-associated signatures can be used to predict how well a patient will do and thus predict outcomes and guide treatment protocols.
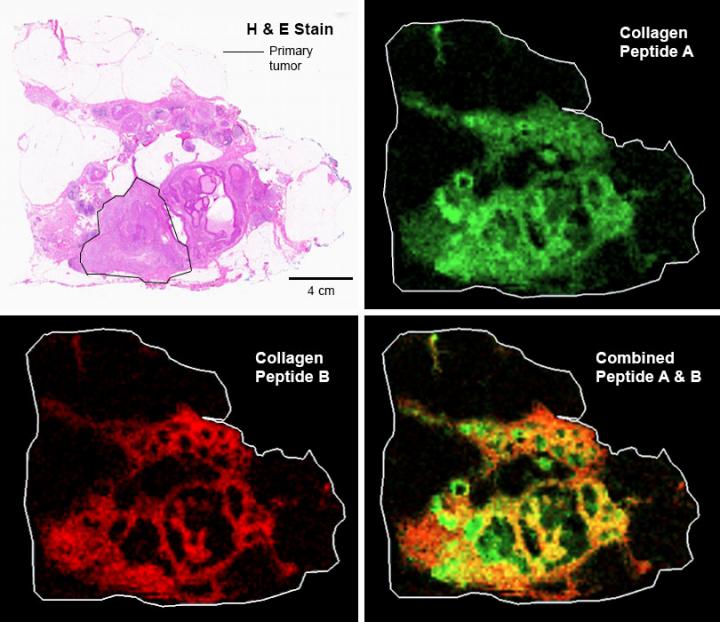
A team of principal investigators at the MUSC Proteomics Center, including Angel, Richard Drake, Ph.D., Anand Mehta, Ph.D., and Lauren Ball, Ph.D., have developed several novel techniques using the specialized analytical capabilities housed within the MUSC Proteomics Center. Angel invented the technique for measuring collagen from formalin-fixed, paraffin-embedded samples. The specific technique she uses is referred to as “matrix-assisted laser desorption ionization imaging mass spectrometry,” or MALDI IMS for short, and allows Angel and her team to profile hundreds to thousands of different chemicals simultaneously, including different types of collagen proteins and their HYP modifications, in patients’ breast cancer tissues.
A different view
Researchers typically have done collagen studies using antibodies or they look at the RNA expression patterns of collagen, so they’re not actually looking at the structure of collagen itself. Her team is taking a new approach, she said.
Her team uses a collagen targeting enzyme and scans the tissue with a mass spectrometer. “At specific X and Y coordinates, called a data pixel, we get signals from hundreds to thousands of collagen peptide peaks. This gives us a map of where collagen changes are happening in breast cancer, such as on the tumor borders or in the tumor itself. We combine these mass spectrometry techniques with microscopy methods to understand how changes in collagen alignment correspond with changes in the actual structure of the collagen. This wasn’t possible before we developed this technique.”
A major benefit of the MALDI IMS approach is its clinical utility, she explained. The mapped molecular markers can be overlaid on top of tissue cancer features identified using more traditional pathological techniques, allowing multiple levels of information to be gathered from the same sample.
“This method fits very well with testing already being done by pathology labs. It could be developed as a test where once a woman gets a lumpectomy, there would then be a tissue section that’s already taken for a pathologist to look at to try to understand what types of cancer cells are there. It will be possible to take that same tissue section and scan it by imaging mass spectrometry and say, ‘This part of your collagen is being modified and that indicates that you may have a need for a more aggressive routine of treatment.'”
The current research award focuses on the defining of changes in the collagen structure that are linked to disparities in breast cancer risk, progression and outcome. While it has not yet been determined what mechanisms drive TNBC collagen changes in African American women, Angel believes these might be linked to certain socioeconomic or lifestyle factors, such as nutrition or reproductive history, an area that she hopes to explore in the future.
Angel anticipates that her novel investigations will lead to far-reaching improvements in breast cancer treatment. She credits support from Hollings, in particular a pilot award from the center’s American Cancer Society Institutional Research Grant, with providing critical preliminary support that led to her current research award from the National Cancer Institute.
“I’m hoping that once we start understanding which collagens contribute to invasive breast cancer, that this is going to be applicable for all women who develop some unique form of invasive breast cancer, and it could possibly be a drug target. There are multiple drug companies that are working on targeting collagens, but their problem is they don’t know exactly what sites are modified and what types of collagens are involved. We think this research will answer some of those questions and increase women’s health overall.”
###
About MUSC
Founded in 1824 in Charleston, MUSC is the oldest medical school in the South, as well as the state’s only integrated, academic health sciences center with a unique charge to serve the state through education, research and patient care. Each year, MUSC educates and trains more than 3,000 students and nearly 800 residents in six colleges: Dental Medicine, Graduate Studies, Health Professions, Medicine, Nursing and Pharmacy. The state’s leader in obtaining biomedical research funds, in fiscal year 2019, MUSC set a new high, bringing in more than $284 million. For information on academic programs, visit musc.edu.
As the clinical health system of the Medical University of South Carolina, MUSC Health is dedicated to delivering the highest quality patient care available, while training generations of competent, compassionate health care providers to serve the people of South Carolina and beyond. Comprising some 1,600 beds, more than 100 outreach sites, the MUSC College of Medicine, the physicians’ practice plan, and nearly 275 telehealth locations, MUSC Health owns and operates eight hospitals situated in Charleston, Chester, Florence, Lancaster and Marion counties. In 2019, for the fifth consecutive year, U.S. News & World Report named MUSC Health the No. 1 hospital in South Carolina. To learn more about clinical patient services, visit muschealth.org.
MUSC and its affiliates have collective annual budgets of $3.2 billion. The more than 17,000 MUSC team members include world-class faculty, physicians, specialty providers and scientists who deliver groundbreaking education, research, technology and patient care.
About MUSC Hollings Cancer Center
MUSC Hollings Cancer Center is a National Cancer Institute-designated cancer center and the largest academic-based cancer research program in South Carolina. The cancer center comprises more than 100 faculty cancer scientists and 20 academic departments. It has an annual research funding portfolio of more than $44 million and a dedication to reducing the cancer burden in South Carolina. Hollings offers state-of-the-art diagnostic capabilities, therapies and surgical techniques within multidisciplinary clinics that include surgeons, medical oncologists, radiation therapists, radiologists, pathologists, psychologists and other specialists equipped for the full range of cancer care, including more than 200 clinical trials. For more information, visit http://www.
Media Contact
Heather Woolwine
[email protected]
Original Source
https:/




