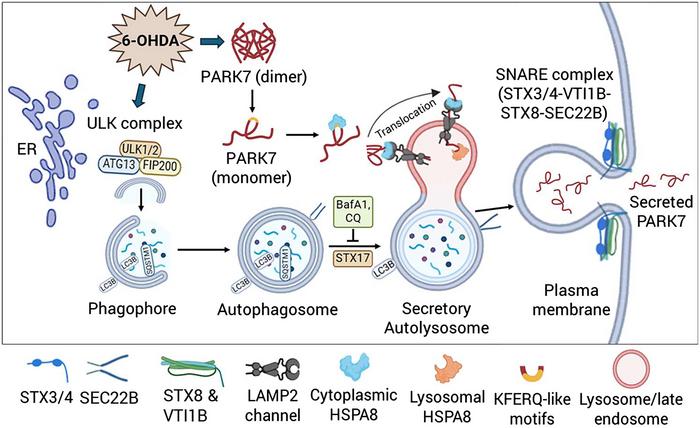
A groundbreaking revelation from researchers at Doshisha University, Japan, has unveiled an extraordinary mechanism underlying the unconventional secretion of PARK7—a protein intricately linked to Parkinson’s disease and cellular oxidative stress responses. This new study illuminates how the interplay between two forms of autophagy, typically regarded as cellular degradation pathways, orchestrates a secretory process divergent from classical protein trafficking. The implications of this discovery herald promising new fronts in understanding neurodegenerative disease biology and potential therapeutic innovation.
Autophagy, a cellular catabolic process originally characterized as a mechanism for cytoplasmic component degradation and recycling, has recently emerged as a key player in unconventional protein secretion. The Doshisha University research team has now demonstrated that PARK7, a Parkinson’s disease-associated protein renowned for its role in antioxidative defense and mitochondrial protection, uses a sophisticated autophagy-based pathway to exit cells under oxidative stress. Their studies reveal that oxidative insults provoked by 6-hydroxydopamine (6-OHDA), a neurotoxin widely utilized to model Parkinsonian neurodegeneration, initiate an orchestrated activation of both macroautophagy and chaperone-mediated autophagy (CMA) that converges on PARK7 secretion.
Intriguingly, PARK7 within these secretory autolysosomes avoids canonical lysosomal degradation, instead being stabilized and transported out of the cell through membrane fusion events mediated by a specialized QabcR-SNARE protein complex comprising STX3/4, VTI1B, STX8, and SEC22B. This unique vesicle trafficking constellation underlines a previously unappreciated dimension of lysosomal function—a dual role in both intracellular catabolism and extracellular secretion under stress conditions, thereby expanding the functional repertoire of autophagy beyond degradation.
Further mechanistic interrogation revealed that disruption of autophagosome-lysosome fusion or inhibition of lysosomal degradation arrested PARK7 secretion, highlighting a critical necessity for functional lysosomal compartments in this process. Moreover, the discovery of SEC22B—an ER-Golgi intermediate compartment R-SNARE—as a key component of the secretory autolysosome fusion machinery provides new molecular detail on the vesicular trafficking steps enabling extracellular delivery of PARK7.
Given PARK7’s importance as a neuroprotective agent, its extracellular role mediated through unconventional secretion pathways may also serve as a novel biomarker avenue for early detection of Parkinson’s disease and related neurodegenerative conditions. This secretory axis could thereby facilitate timely diagnosis and intervention, fostering improved clinical outcomes.
The authors emphasize the broader implications of this secretory mechanism, suggesting that adaptive cellular responses to stress can repurpose degradative organelles like lysosomes for trafficking and secretion functions, adding complexity to autophagic biology. This paradigm shift underscores the need for further exploration of autophagy’s secretory capabilities and their influence on cellular homeostasis and disease etiology.
Looking ahead, the research team aspires that their findings will invigorate drug development aimed at secretory autolysosome pathways, enhancing cellular resilience to oxidative insults, and bolstering lysosomal function. Such therapeutic strategies hold promise for neurodegenerative diseases, where aberrant protein handling and oxidative stress converge to drive pathology.
This pioneering work, authored by Dr. Biplab Kumar Dash, Professor Yasuomi Urano, and Professor Noriko Noguchi—respected scientists from Doshisha University—solidifies their continuing contribution to unraveling the intersection of autophagy and unconventional protein secretion, setting a new standard for molecular research in neurodegeneration.
—
Subject of Research: Cells
Article Title: Unconventional secretion of PARK7 requires lysosomal delivery via chaperone-mediated autophagy and specialized SNARE complex
News Publication Date: 6-May-2025
Web References:
https://www.pnas.org/doi/10.1073/pnas.2414790122
Image Credits: Dr. Biplab Kumar Dash from Doshisha University, Japan
Keywords: Lysosomal function, Cancer, Gene transcription, Oxidative stress, Parkinsons disease
Tags: autophagy in neurodegenerationcellular oxidative stress responsesDoshisha University research findingsendoplasmic reticulum and Golgi apparatuseukaryotic cell protein secretionintracellular vesicle exocytosisleaderless proteins in secretionlysosomal pathways in protein traffickingmechanisms of autophagy and secretionPARK7 protein and Parkinson’s diseasetherapeutic innovations for neurodegenerative diseasesunconventional protein secretion mechanisms