Credit: Copyright 2020 AANS.
CHARLOTTESVILLE, VA (DECEMBER 1, 2020). Researchers from multiple institutions in North America have developed a fully automated, deep-learning (DL), artificial-intelligence clinical tool that can measure the volume of cerebral ventricles on magnetic resonance images (MRIs) in children within about 25 minutes. The ability to track ventricular volume over time in a clinical setting will prove invaluable in the treatment of children and adults with hydrocephalus. Details on the development of the tool and its validation are reported today in a new article, “Artificial intelligence for automatic cerebral ventricle segmentation and volume calculation: a clinical tool for the evaluation of pediatric hydrocephalus,” by Jennifer L. Quon, MD, and colleagues, in the Journal of Neurosurgery: Pediatrics .
Hydrocephalus is a pathological condition caused by an excessive amount of cerebrospinal fluid (CSF) in chambers of the brain known as ventricles. The condition results from an imbalance between the production and absorption of CSF. Hydrocephalus is called “communicating” when CSF can pass from one ventricle to another and “obstructive” when passage from one ventricle to another is blocked. The prevalence of pediatric hydrocephalus is approximately six in 10,000 live births. It has been called “the most common surgically correctable neurological problem in infants, children, and adolescents.”
Diagnosis of hydrocephalus is based on clinical signs and symptoms as well as on findings of enlarged ventricles on neuroimaging studies. Placement of a shunt (an internal draining system that drains excess CSF away from the brain) is the most common surgical procedure performed to reduce hydrocephalus. Following surgery, patients must be monitored periodically to ensure that the shunt continues to work properly. Changes in ventricular volume can guide clinical decision-making. However, to date, accurate assessments of ventricular volume can be time consuming or require research-level automated tools that are not easily adapted to the patient’s clinical visit.
The authors of this study sought to develop an automated deep-learning (DL)-based model that could be used to evaluate changes in the volume of brain ventricles over time in children with hydrocephalus during their clinic visits. Deep learning is an advanced form of artificial intelligence that mimics the workings of the human brain; it is capable of processing large quantities of data and creating patterns used in decision making. The authors’ goal was to create a DL tool that would work efficiently in multiple institutions with various clinical MRI machines from different manufacturers.
To develop and validate the model, the authors selected sets of T2-weighted MRIs from a group of 200 pediatric patients (22 years of age or younger) who had presented with acute obstructive hydrocephalus. T2-weighted MRIs have wide clinical use but are not usually used to determine ventricular volume. The patients in this group had been treated at one of four institutions: Lucile Packard Children’s Hospital Stanford; Seattle Children’s Hospital; The Hospital for Sick Children; and Dayton Children’s Hospital. For a control group, the authors selected 200 sets of T2-weighted MRIs from 199 neurologically intact pediatric patients. Three-dimensional T1-weighted MRIs, which had been obtained in all controls and a subset of patients with hydrocephalus, were also reviewed. Three-dimensional T1-weighted MRIs are commonly used for volumetric analysis but are not readily available in the clinic.
The 400 sets of T2-weighted MRIs were separated for use in various steps of the study: training (266 MRI sets) and optimization (67 MRI sets) of the DL model, and a held-out test (67 MRI sets) for final evaluation of the model’s performance. In a separate study, the authors also studied the generalizability of the DL model and its clinical usefulness using T2-weighted MRIs that were prospectively obtained in nine patients at Utah Primary Children’s Hospital.
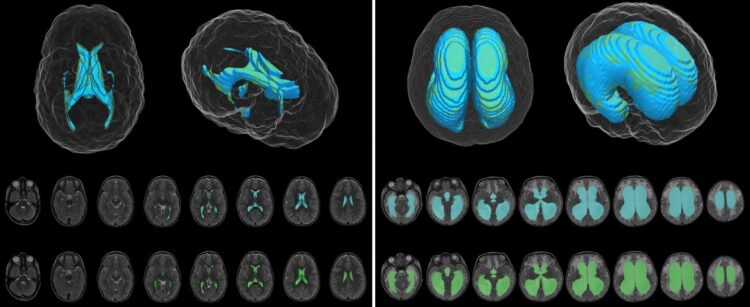
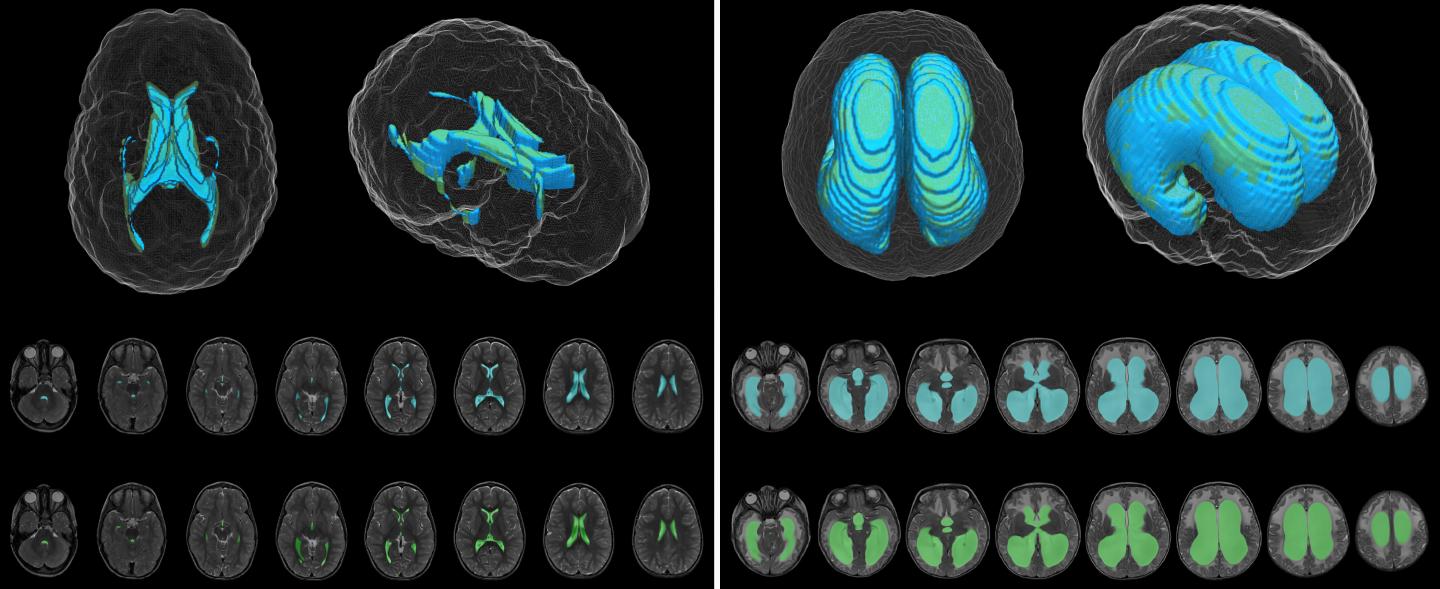
The DL model was designed to produce automatic ventricle segmentation (delineation of ventricle borders on imaging) and volume calculation. To examine the efficiency of the model, the authors compared these two processes to the gold standard of manual segmentation and volume calculation and to the use of FreeSurfer research software. The authors used the Dice similarity coefficient (0 to 1) to assess segmentation accuracy and linear regression to assess volume calculation.
According to the authors, when compared to manual segmentation, “model segmentation performed with an overall Dice score of 0.901 (0.946 in hydrocephalus, 0.856 in controls).” These numbers show great accuracy, with even better accuracy evident when used in patients with hydrocephalus. When used to assess segmentation accuracy in the patients at Utah Primary Children’s Hospital, the Dice score was 0.926.
The authors found a strong correlation between ventricular volume calculations made using the DL model and the manually determined frontal-occipital horn ratio (r2 = 0.92) and Evans’ index, a frontal horn ratio (r2 = 0.79). These calculations were made using T2-weighted MRIs.
The DL model was more accurate and much faster than FreeSurfer software, which “took 8.2 to 207.3 hours (median 20.3 hours) for ventricle segmentation and volume output, compared with 1.48 seconds per patient scan for the DL model.”
This work is still preliminary. Evidence provided using the DL model still requires correlation with patients’ symptoms, and more work needs to be done to evaluate the DL model when used with other types of hydrocephalus. Nevertheless, the authors conclude, “With near-immediate volumetric output and reliable performance across institutional scanner types, this model can be adapted to the real-time clinical evaluation of hydrocephalus and improve clinician workflow.”
When asked about the findings of the study, Drs. Edwards and Yeom responded, “It has been more than 100 years since Dandy developed ventriculography to visualize the ventricular system. Our goal was to develop a rapid, reliable program using AI [artificial intelligence] Technology that is fast, accurate, and deployable across multiple imaging platforms. Having definitive ventricular volumes will remove the labor and inaccuracy in measuring and comparing ventricular size over time and should allow more accurate decisions in managing patients with hydrocephalus and other CSF volume pathologies. Our goal moving forward is to validate our technique clinically in order to move this technique into routine clinical and research use. Our hope is that this technology will provide more accurate and reliable information to allow clinicians to make better management decisions in patients with hydrocephalus and thereby improve patient care and outcomes.”
###
Quon JL, Han M, Kim LH, Koran ME, Chen LC, Lee EH, Wright J, Ramaswamy V, Lober RM, Taylor MD, Grant GA, Cheshier SH, Kestle JRW, Edwards MSB, Yeom KW: Artificial intelligence for automatic cerebral ventricle segmentation and volume calculation: a clinical tool for the evaluation of pediatric hydrocephalus. Journal of Neurosurgery: Pediatrics, published online, ahead of print, December 1, 2020; DOI: 10.3171/2020.6.PEDS20251.
Jennifer L. Quon, Michelle Han, Michael S. B. Edwards, and Kristen W. Yeom contributed equally to this work.
Drs. Quon, Han, Kim, Koran, and Chen are affiliated with Stanford University School of Medicine, and Dr. Lee is affiliated with Stanford University School of Engineering. Dr. Wright is affiliated with Seattle Children’s Hospital, University of Washington School of Medicine; Drs. Ramaswamy and Taylor with The Hospital for Sick Children, University of Toronto; Dr. Lober with Dayton Children’s Hospital, Wright State University Boonshoft School of Medicine; Drs. Cheshier and Kestle with the University of Utah School of Medicine; and Drs. Grant, Edwards, and Yeom with both Stanford University School of Medicine and Lucile Packard Children’s Hospital Stanford, Stanford, California.
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
For additional information, please contact: Ms. Jo Ann M. Eliason, Communications Manager, Journal of Neurosurgery Publishing Group, One Morton Drive, Suite 200, Charlottesville, VA 22903.
Email: [email protected] Phone: 434-982-1209
The Journal of Neurosurgery: Pediatrics is a monthly peer-reviewed journal focused on diseases and disorders of the central nervous system and spine in children. This journal contains a variety of articles, including descriptions of preclinical and clinical research as well as case reports and technical notes. The Journal of Neurosurgery: Pediatrics is one of five journals published by the JNS Publishing Group, the scholarly journal division of the American Association of Neurological Surgeons. Other peer-reviewed journals published by the JNS Publishing Group include the Journal of Neurosurgery, Journal of Neurosurgery: Spine, Neurosurgical Focus, and Neurosurgical Focus: Video. All five journals can be accessed at http://www.
Founded in 1931 as the Harvey Cushing Society, the American Association of Neurological Surgeons (AANS) is a scientific and educational association with more than 10,000 members worldwide. The AANS is dedicated to advancing the specialty of neurological surgery in order to provide the highest quality of neurosurgical care to the public. All active members of the AANS are certified by the American Board of Neurological Surgery, the Royal College of Physicians and Surgeons (Neurosurgery) of Canada, or the Mexican Council of Neurological Surgery, AC. Neurological surgery is the medical specialty concerned with the prevention, diagnosis, treatment, and rehabilitation of disorders that affect the entire nervous system including the brain, spinal column, spinal cord, and peripheral nerves. For more information, visit http://www.
Media Contact
Jo Ann M Eliason
[email protected]
Related Journal Article
http://dx.