University of Tsukuba researchers develop high-throughput screening for factors promoting cardiac reprogramming, and identify a non-steroidal anti-inflammatory drug as one such factor in postnatal and adult fibroblasts
Credit: University of Tsukuba
Tsukuba, Japan – Once damaged, the human heart does a poor job of repairing itself, so this is a key priority for treating heart failure. One way of restoring cardiac function is to reprogram non-cardiac body cells such as fibroblasts into heart muscle cells (cardiomyocytes) using a collection of cardiac transcription factors.
This bypasses the need to use stem cells as an intermediate and avoids stimulating the proliferation of existing cardiomyocytes. However, the reprogramming of postnatal and adult fibroblasts is inefficient compared with that of embryonic fibroblasts, and the challenges associated with reprogramming aged cells are unclear.
A new study in Nature Communications has advanced this field by using a high-throughput screening approach to identify diclofenac, an FDA-approved drug commonly used to treat inflammation and rheumatic diseases, as a factor promoting cardiac reprogramming in postnatal and adult fibroblasts but not embryonic ones. The finding by researchers at the University of Tsukuba and their research colleagues in a Japan-wide university collaboration also helps define the barriers unique to cellular aging.
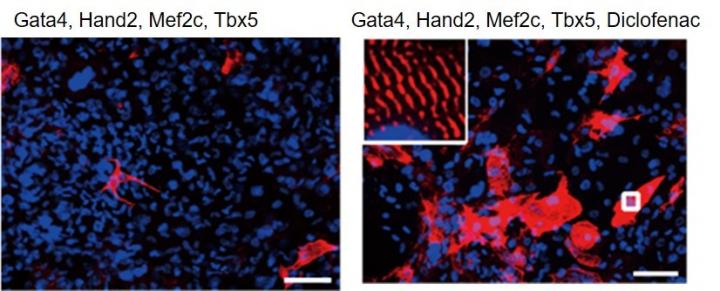
Previous methods of identifying cardiac reprogramming factors have been labor intensive and unsuitable for large-scale screening. “As an alternative approach, we developed a high-content technique to screen a chemical library of 8400 compounds,” says co-author Taketaro Sadahiro. “The first round of screening identified 37 potential compounds, which were narrowed down to four in the second round. The most powerful of these four molecules was diclofenac.”
Based on these findings, diclofenac was shown to improve cardiac reprogramming in a dose-dependent manner largely by inhibiting the enzyme COX-2, which is highly expressed in postnatal and adult fibroblasts. Diclofenac also suppressed a host of signaling molecules, including several mediators of inflammation.
The team found that diclofenac functioned during the early stages of cardiac reprogramming, and increased the generation of cardiomyocytes more quickly and efficiently than TGF? and Wnt inhibitors, which are factors known to promote reprogramming.
“Consistent with the importance of inflammation in preventing cardiac reprogramming, our microarray data showed that diclofenac downregulated fibroblast and inflammatory genes, and upregulated cardiac genes,” says corresponding author Masaki Ieda. “Thus, diclofenac was responsible for silencing fibroblast signatures, which occurred prior to cellular reprogramming into cardiomyocytes.”
As well as COX-2, genes associated with inflammation and fibroblasts were also shown to be more highly expressed in postnatal and adult cells compared with embryonic fibroblasts. This indicated that inflammation and fibrogenesis are age-related barriers to cardiac reprogramming. These findings have important implications for the development of new therapies for cardiac regeneration in pediatric and adult patients.
###
Media Contact
Masataka Watanabe
[email protected]
Related Journal Article
http://dx.




