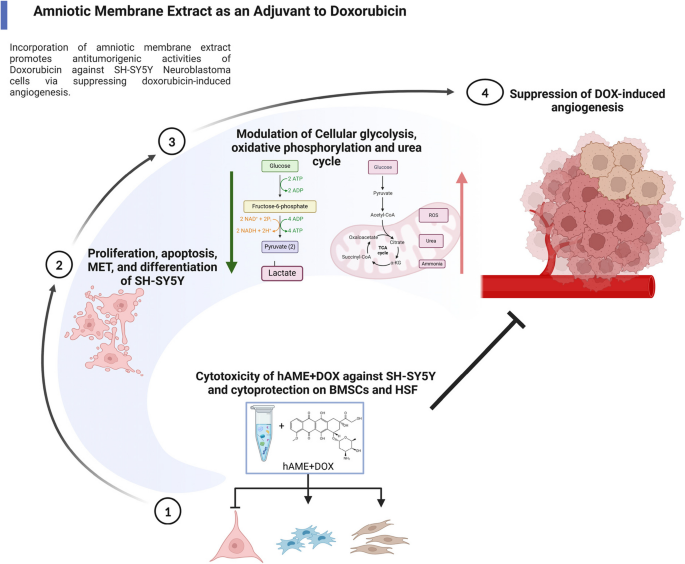
In a groundbreaking new study published in BMC Cancer, researchers unveil a promising adjuvant therapy that significantly enhances the efficacy of doxorubicin (DOX) in treating neuroblastoma (NB), a devastating pediatric cancer. The research team discovered that extracts from the human amniotic membrane (hAME) can potentiate DOX’s cancer-fighting capabilities by inhibiting the angiogenesis process in SH-SY5Y neuroblastoma cells. This novel combination offers hope for improved therapeutic outcomes with potentially reduced side effects, setting the stage for more targeted and effective cancer treatment strategies.
Human amniotic membrane extracts, rich in a complex mixture of proteins and bioactive molecules, have gained increasing attention for their intrinsic anti-cancer and anti-angiogenic properties. The current study sought to dissect the therapeutic potential of hAME when paired with DOX, hypothesizing that hAME could counteract DOX-induced angiogenesis and offer a multimodal approach to shutting down NB tumor growth and vascularization.
.adsslot_YwoVaXMnNh{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_YwoVaXMnNh{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_YwoVaXMnNh{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Using a suite of advanced cellular, molecular, and biochemical assays, the researchers meticulously studied the effects of the DOX and hAME combination—referred to as D+E treatment—on several pivotal hallmarks of neuroblastoma progression. They assessed parameters such as cell proliferation rates, cell cycle dynamics, angiogenesis indices, invasiveness, differentiation state, and bioenergetic profiles of SH-SY5Y cells, a widely used human neuroblastoma cell line.
Beyond cell growth inhibition, the combined therapy also antagonized the invasive capabilities of neuroblastoma cells, which are critical for metastasis and disease spread. The treatment promoted a mesenchymal-to-epithelial transition (MET), a differentiation shift typically associated with reduced malignancy and restored cell adhesion properties. Such phenotypic reprogramming could hinder the likelihood of tumor dissemination, further underscoring the clinical relevance of the approach.
Cellular bioenergetics also underwent a remarkable shift upon D+E treatment. The researchers observed a halt in glycolytic metabolism, often exploited by aggressive cancer cells for energy production, indicative of what is known as the Warburg effect. Concurrently, data suggest a possible shift toward oxidative phosphorylation and enhanced urea cycle activity, metabolic pathways linked to healthier cellular function and reduced tumorigenic potential. This metabolic reprogramming may underpin the observed anti-cancer effects and enhance cellular vulnerability to chemotherapy.
Crucially, mechanistic studies revealed that hAME effectively abrogates the pro-angiogenic response induced by DOX. Angiogenesis, a process essential for tumor growth and nutrient supply, was significantly curtailed, as demonstrated by in vitro models and corroborated by in vivo experiments using a chick embryo assay. The inhibition of vessel formation points to a vital role for hAME in normalizing tumor vasculature and preventing the establishment of new blood supply routes that tumors rely on for survival.
The suppression of angiogenesis was linked mechanistically to the downregulation of the PHD-2/HIF-1α axis, a pathway already implicated in DOX’s paradoxical effects. By modulating this molecular circuitry, hAME restores the balance between anti-angiogenic and pro-angiogenic signals, thereby transforming DOX treatment from a double-edged sword into a more precise anti-cancer weapon.
These insights not only deepen our understanding of the complex interactions between chemotherapy agents and tumor biology but also showcase the therapeutic potential of leveraging naturally derived biological extracts in combinatorial regimens. The dual action of hAME—targeting both cancer cell survival and the tumor microenvironment—may provide a blueprint for designing future adjuvant therapies that amplify efficacy while minimizing systemic toxicity.
This study presents a compelling argument for the advancement of hAME as an adjunct to conventional chemotherapy, with the promise of delaying or even circumventing the development of drug resistance. As resistance to DOX remains a significant hurdle in NB management, therapies that disrupt the pro-tumorigenic countermeasures elicited by chemotherapy are of paramount importance.
Moving forward, the translation of these findings into clinical contexts warrants rigorous in vivo validation in mammalian models, dosage optimization, and safety assessments. Further exploration into the specific components of hAME responsible for its anti-angiogenic properties could open doors to purified or synthetic derivatives that provide a consistent therapeutic effect. Additionally, the impact of hAME on other cancer subtypes that similarly exploit angiogenesis as a growth mechanism merits investigation.
In conclusion, the combination of doxorubicin and human amniotic membrane extract represents a multifaceted therapeutic strategy capable of targeting neuroblastoma cells across multiple biological dimensions. Through synergistic inhibition of proliferation, invasiveness, and angiogenesis, coupled with beneficial effects on cellular metabolism and differentiation, this approach could redefine treatment paradigms for one of the most challenging pediatric cancers. The promise of such biologically inspired adjuvant therapies aligns with the ongoing quest for more effective, less harmful interventions against cancer.
As our understanding of tumor biology evolves, integrating naturally derived biomaterials like hAME with existing chemotherapeutics exemplifies the innovative avenues available for combating resistant cancers. The potential for reduced side effects and enhanced outcomes could translate into improved survival rates and quality of life for affected children, marking a significant step forward in oncologic therapeutics.
With the publication of these findings in BMC Cancer, the research team invites the scientific and medical communities to explore this novel therapeutic axis further. Collaborative efforts spanning basic research, clinical trials, and pharmacological development will be essential to realize the full potential of this promising treatment.
The future of neuroblastoma therapy may very well lie in combining the precision of modern chemotherapy with the subtle biological activity of natural extracts, creating a powerful synergy that redefines how we approach cancer treatment.
Subject of Research: Neuroblastoma treatment enhancement via combination therapy using doxorubicin and human amniotic membrane extract targeting tumor angiogenesis and progression.
Article Title: Amniotic membrane promotes doxorubicin potency by suppressing SH-SY5Y neuroblastoma cell angiogenesis.
Article References:
Abou-Shanab, A.M., Shouman, S., Hussein, A.E. et al. Amniotic membrane promotes doxorubicin potency by suppressing SH-SY5Y neuroblastoma cell angiogenesis. BMC Cancer 25, 1021 (2025). https://doi.org/10.1186/s12885-025-14442-z
Image Credits: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14442-z
Tags: adjuvant therapy for neuroblastomaamniotic membrane therapyanti-angiogenic properties of hAMEdoxorubicin neuroblastoma treatmentenhancing chemotherapy efficacyneuroblastoma treatment strategiesnew cancer treatment combinationspediatric cancer therapiesPHD-2 HIF-1α signaling pathwayreducing side effects of doxorubicinSH-SY5Y neuroblastoma cell studiestherapeutic outcomes in cancer