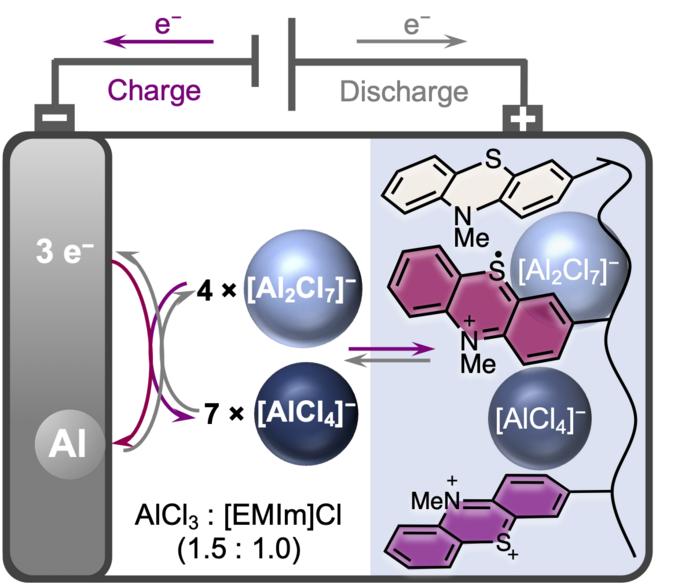
Aluminium-ion batteries are seen as a promising alternative to conventional batteries that use scarce and difficult-to-recycle raw materials such as lithium. This is because aluminium is one of the most common elements in the Earth’s crust, is easier to recycle, and is also safer and less expensive than lithium. However, the development of such aluminium-ion batteries is still in its infancy, as suitable electrode materials that provide sufficient storage capacity are still lacking. A research team headed by Gauthier Studer and led by Prof. Dr. Birgit Esser of the University of Ulm and Prof. Dr. Ingo Krossing as well as Prof. Dr. Anna Fischer of the University of Freiburg has now developed a positive electrode material consisting of an organic redox polymer based on phenothiazine. In the experiment, aluminium batteries with this electrode material stored a previously unattained capacity of 167 milliampere hours per gram (mAh/g). The organic redox polymer thus surpasses the capacity of graphite, which has mostly been used as an electrode material in batteries to date. The results appeared in the journal Energy & Environmental Science.
Credit: Birgit Esser / University of Freiburg
Aluminium-ion batteries are seen as a promising alternative to conventional batteries that use scarce and difficult-to-recycle raw materials such as lithium. This is because aluminium is one of the most common elements in the Earth’s crust, is easier to recycle, and is also safer and less expensive than lithium. However, the development of such aluminium-ion batteries is still in its infancy, as suitable electrode materials that provide sufficient storage capacity are still lacking. A research team headed by Gauthier Studer and led by Prof. Dr. Birgit Esser of the University of Ulm and Prof. Dr. Ingo Krossing as well as Prof. Dr. Anna Fischer of the University of Freiburg has now developed a positive electrode material consisting of an organic redox polymer based on phenothiazine. In the experiment, aluminium batteries with this electrode material stored a previously unattained capacity of 167 milliampere hours per gram (mAh/g). The organic redox polymer thus surpasses the capacity of graphite, which has mostly been used as an electrode material in batteries to date. The results appeared in the journal Energy & Environmental Science.
Electrode material inserts complex aluminium anions
The electrode material is oxidized during charging of the battery, thereby taking up complex aluminate anions. In this way, the organic redox polymer poly(3-vinyl-N-methylphenothiazine) manages to insert two [AlCl4]− anions reversibly during charging. The researchers used the ionic liquid ethylmethylimidazolium chloride as electrolyte with added aluminium chloride. “The study of aluminium batteries is an exciting field of research with great potential for future energy storage systems,” says Gauthier Studer. “Our focus lies on developing new organic redox-active materials that exhibit high performance and reversible properties. By studying the redox properties of poly(3-vinyl-N-methylphenothiazine) in chloroaluminate-based ionic liquid, we have made a significant breakthrough by demonstrating for the first time a reversible two-electron redox process for a phenothiazine-based electrode material.”
After 5,000 charge cycles at 10 C, battery retains 88 percent of its capacity
Poly(3-vinyl-N-methylphenothiazine) deposits the [AlCl4]− anions at potentials of 0.81 and 1.65 volts and provides specific capacities of up to 167 mAh/g. In contrast, the discharge capacity of graphite as electrode material in aluminium batteries is 120 mAh/g. After 5,000 charge cycles, the battery presented by the research team still has 88 percent of its capacity at 10 C, i.e. at a charge and discharge rate of 6 minutes. At a lower C rate, i.e. a longer charge and discharge time, the battery returns unchanged to its original capacities.
“With its high discharge voltage and specific capacity, as well as its excellent capacity retention at fast C rates, the electrode material represents a major advance in the development of rechargeable aluminium batteries and thus of advanced and affordable energy storage solutions,” says Birgit Esser.
Overview of facts:
- Original publication: G. Studer, A. Schmidt, J. Büttner, M. Schmidt, A. Fischer, I. Krossing and B. Esser, On a high-capacity aluminium battery with a two-electron phenothiazine redox polymer as positive electrode, Energy Environ. Sci., 2023, DOI: 10.1039/D3EE00235G
- Gauthier Studer is pursuing his doctorate at the Institute for Organic Chemistry II and Advanced Materials at the University of Ulm.
- Prof. Dr. Birgit Esser holds the Chair of Organic Chemistry at the Institute of Organic Chemistry II and Advanced Materials at the University of Ulm. She is a member of the Clusters of Excellence Post Lithium Storage (POLiS) at the University of Ulm and Living, Adaptive and Energy-autonomous Materials Systems (livMatS) at the University of Freiburg.
- Prof. Ingo Krossing holds the Chair of Molecular- and Coordination Chemistry at the Institute of Inorganic and Analytical Chemistry at the University of Freiburg and is a member of the Cluster of Excellence Living, Adaptive and Energy-autonomous Materials Systems (livMatS).
- Prof. Dr. Anna Fischer holds the Chair for Functional Inorganic Materials at the Institute of Inorganic and Analytical Chemistry at the University of Freiburg and is spokesperson for the livMatS Cluster of Excellence.
- The project was funded by the German Research Foundation (DFG) (project AMPERE within SPP 2248 – Polymer-based Batteries, POLiS – EXC 2154, livMatS – EXC 2193) as well as by the Deutsche Bundesstiftung Umwelt, the bwForCluster JUSTUS 2, the Eva Mayr-Stihl-Stiftung (Saltus!) and the Land Baden-Württemberg (bwHCP).
Journal
Energy & Environmental Science
DOI
10.1039/D3EE00235G




