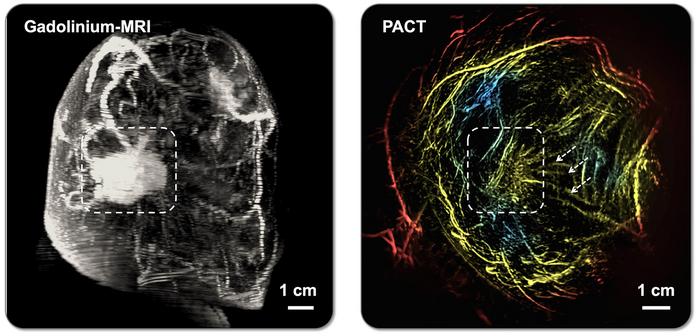
A groundbreaking advancement in breast cancer detection has emerged from a team of researchers led by Caltech, unveiling an innovative imaging technique that promises to transform diagnostic practices. This novel method harnesses the power of photoacoustic computed tomography (PACT), an approach that combines the penetrating capabilities of sound with the molecular sensitivity of light, providing an imaging experience that is not only safer and more comfortable for patients but also potentially more precise than conventional methods. Importantly, this technique integrates sophisticated machine learning algorithms to enhance its ability to distinguish between malignant and benign tissue, signaling a significant leap forward in breast cancer diagnostics.
For decades, mammography has served as the frontline tool for early breast cancer detection. Despite its invaluable role in saving lives, mammography is fraught with challenges that limit its efficacy and patient acceptance. The process involves compressing the breast between plates, causing discomfort and anxiety for many, while exposing patients to low doses of ionizing radiation. Additionally, the technique’s reliability diminishes markedly in patients with dense breast tissue, leading to increased false-positive rates and unnecessary biopsies. Amid efforts to improve cancer detection, alternatives like ultrasound and magnetic resonance imaging have been employed but do not perfectly address these limitations. Ultrasound’s accuracy depends heavily on the operator’s expertise and often yields inconclusive results, whereas MRI, though highly sensitive, is costly, time-consuming, and unsuitable for patients with allergies to contrast agents or claustrophobia.
The Caltech team, motivated by these unmet clinical needs, has spent over two decades refining PACT, a method that converges optical and acoustic imaging into a single, non-invasive modality. PACT deploys short pulses of near-infrared laser light into the breast tissue, which are absorbed by molecules like hemoglobin in red blood cells. This absorption triggers ultrasonic vibrations as the molecules rapidly expand and contract. Unlike traditional imaging that relies solely on ionizing radiation or sound waves, PACT measures these vibrations using a dense array of ultrasonic sensors surrounding the breast, reconstructing high-resolution images of the internal tissue architecture.
.adsslot_421bfC7l5G{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_421bfC7l5G{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_421bfC7l5G{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
This dual nature of PACT offers distinctive advantages. While light scattering in biological tissues typically limits optical imaging resolution, the acoustic signals generated by molecular vibrations travel with less scattering, preserving spatial accuracy. This synergy allows PACT to achieve exceptional resolution, capturing structures as small as 0.25 millimeters deep within 4 centimeters of tissue. PACT’s ability to visualize oxygen-rich blood vessels, indicative of angiogenesis commonly seen in tumors, and detect hypoxic regions within tumors—areas starved of oxygen due to rapid cellular metabolism—provides unique physiological insights that exceed the scope of conventional imaging.
Integrating machine learning into PACT has further enhanced its diagnostic potential. The system has been trained on a diverse dataset of breast tissue images, enabling it to recognize subtle differences that may elude even experienced radiologists. By learning complex patterns associated with malignancy or benignity, the machine learning model improves the sensitivity and specificity of tumor detection. It can identify early signs of pathological changes, sometimes preceding visible abnormalities in traditional imagery, which opens avenues for earlier intervention and improved patient outcomes.
In practical terms, the PACT system is designed with patient comfort at the forefront. Scanning involves the patient lying prone on a cushioned table with a recessed area containing warm water and the sensor array, where one breast at a time is immersed. Laser pulses are emitted beneath the breast, and rapid data acquisition completes each scan in as little as 15 seconds—short enough for patients to hold their breath briefly, minimizing motion artifacts. This fast, painless procedure stands in stark contrast to the discomfort and duration of mammograms or MRI sessions, potentially improving patient compliance and screening rates.
Clinical validation at the City of Hope Comprehensive Cancer Center involved testing PACT on 39 patients, yielding results on par with, or exceeding, those obtained by mammography and MRI in distinguishing suspicious from normal tissue and classifying lesions as malignant or benign. These promising results signal a path toward wider clinical adoption and could initiate a paradigm shift in how breast cancer screening and diagnosis are performed.
Despite the substantial progress, the research team envisions further refinements. Future iterations of PACT aim to incorporate multiple laser wavelengths to discern different molecular signatures, potentially expanding the array of detectable tissue characteristics. Additionally, expanding training datasets from larger patient populations will improve the robustness and accuracy of the machine learning algorithms. The ultimate goal is to translate this technology into an affordable, clinically practical tool accessible to entire populations at risk.
The research, detailed in a recent article in Nature Biomedical Engineering, is the culmination of collaborative efforts from graduate students, postdoctoral researchers, and clinical experts. Their paper, titled “Panoramic photoacoustic computed tomography with learning-based classification enhances breast lesion characterization,” outlines the technical foundations and the clinical impacts of the technology. Funding support from national health agencies underscores the significance of this innovation in public health and medical imaging.
By bridging the gap between molecular imaging and acoustic detection, this breakthrough technology offers a vision of breast cancer detection that is swift, safe, accurate, and patient-friendly. It stands as a testament to the fusion of optical physics, engineering, and artificial intelligence, heralding a new era in diagnostic medicine where metabolic and physiological information guides cancer detection more effectively than ever before.
Subject of Research: Breast cancer imaging using photoacoustic computed tomography integrated with machine learning for improved lesion characterization.
Article Title: Panoramic photoacoustic computed tomography with learning-based classification enhances breast lesion characterization
News Publication Date: 24-Jun-2025
Web References:
Article DOI: 10.1038/s41551-025-01435-3
Caltech news: Laser-sonic scanner aims to replace mammograms finding breast cancer
References: Scientific paper published in Nature Biomedical Engineering, with contributions from Caltech and City of Hope Comprehensive Cancer Center researchers.
Image Credits: Xin Tong/Caltech Optical Imaging Laboratory
Keywords: Medical imaging, Mammography, Breast cancer
Tags: advanced breast cancer detectionAI-powered breast imagingalternatives to mammographybreast tissue analysisimproving cancer detection methodsinnovative diagnostic practicesmachine learning in diagnosticspainless breast cancer screeningpatient comfort in imagingphotoacoustic computed tomographyreducing false-positive ratessafer imaging techniques