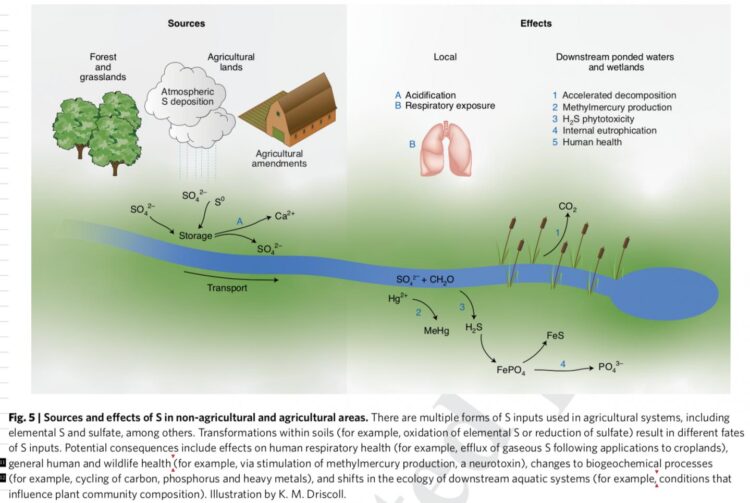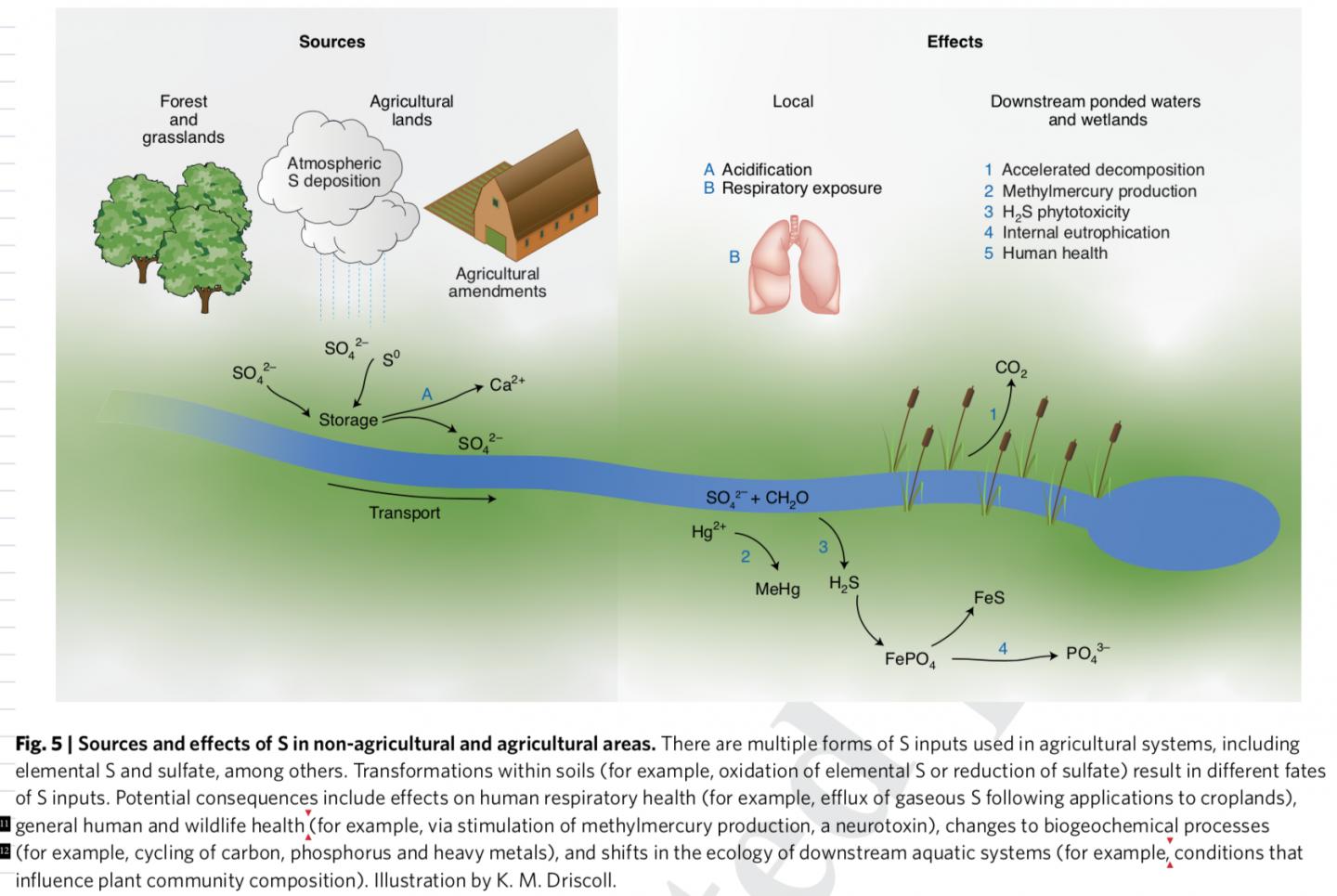
Credit: Illustration by K. M. Driscoll
Historically, coal-fired power plants were the largest source of reactive sulfur, a component of acid rain, to the biosphere. A new study recently publishing Aug. 10 in the journal Nature Geoscience shows that fertilizer and pesticide applications to croplands are now the most important source of sulfur to the environment.
Acid rain gained attention in the 1960s and 1970s when scientists linked degradation of forest and aquatic ecosystems across the northeastern US and Europe to fossil fuel emissions from industrial centers often hundreds of kilometers away. This research prompted the Clean Air Act and its Amendments, which regulated air pollution, driving sulfur levels in atmospheric deposition down to low levels today.
“It seemed like the sulfur story was over,” said Eve-Lyn Hinckley, assistant professor of environmental studies at University of Colorado, Boulder, and lead author of the study. “But our analysis shows that sulfur applications to croplands in the US and elsewhere are often ten times higher than the peak sulfur load in acid rain. No one has looked comprehensively at the environmental and human health consequences of these additions.”
Sulfur is a naturally occurring element that exists primarily in stable, geologic forms and is an important plant nutrient. Through mining activities, including fossil fuel extraction as well as synthesis of fertilizers and pesticides, sulfur is brought into air, land, and water systems. It can react quickly, and, as decades of research on acid rain showed, affect ecosystem health and the cycling of toxic metals that pose a danger to wildlife and people.
“Although sulfur is applied to agricultural lands to improve the production and health of crops, it can have detrimental effects to agricultural soils and downstream waters, similar to what occurred in remote forest landscapes under acid rain,” indicates Charles Driscoll, a professor at Syracuse University and co-author of the study.
The researchers examined trends in sulfur applications across multiple important crops in the US, including corn in the Midwest, sugarcane in Florida, and wine grapes in California. Their models of surface water sulfate export demonstrate that while areas like New England show declining trends in response to recovery from historic atmospheric deposition, sulfate export from agricultural areas is increasing.
Driscoll says an example of the impacts of agricultural applications of sulfur is the enhanced formation of methylmercury in waters draining agricultural lands, such as the Everglades Agricultural Area in Florida. Methylmercury is a potent neurotoxin which accumulates in food chains leading to high concentrations in fish and increasing exposure of mercury to humans and wildlife that consume these fish.
The researchers predict that increasing trends will continue in many croplands around the world, including places like China and India that are still working to regulate fossil fuel emissions.
To date, much research has focused on understanding and regulating nitrogen and phosphorus fertilizers, which can cause eutrophication, fish kills, and harmful algal blooms downstream of agricultural areas.
Hinckley and Driscoll believe it is time for the research community to apply lessons learned while investigating the effects of nitrogen and phosphorus fertilizers to studying the implications of high sulfur use in agriculture. This research must seek not only to document its environmental and human health effects, but also to collaborate with farmers to investigate how to optimize sulfur use.
“Sulfur in agriculture is not going away,” said Hinckley, “Yet there is an opportunity to bring science and practice together to create viable solutions that protect long-term environmental, economic, and human health goals.”
Researchers from the University of Colorado, Boulder, University of Southern Illinois at Carbondale, and Syracuse University participated in this study.
###
Media Contact
Daryl Lovell
[email protected]
Related Journal Article
http://dx.