‘These www.Aging-US.com results suggest an alteration in DNA replication/repair function of POLD1 as a primary pathogenetic cause of MDPL.’
Credit: Correspondence to: Maria Rosaria D’Apice email: [email protected] and Antonella Sgura email: [email protected]
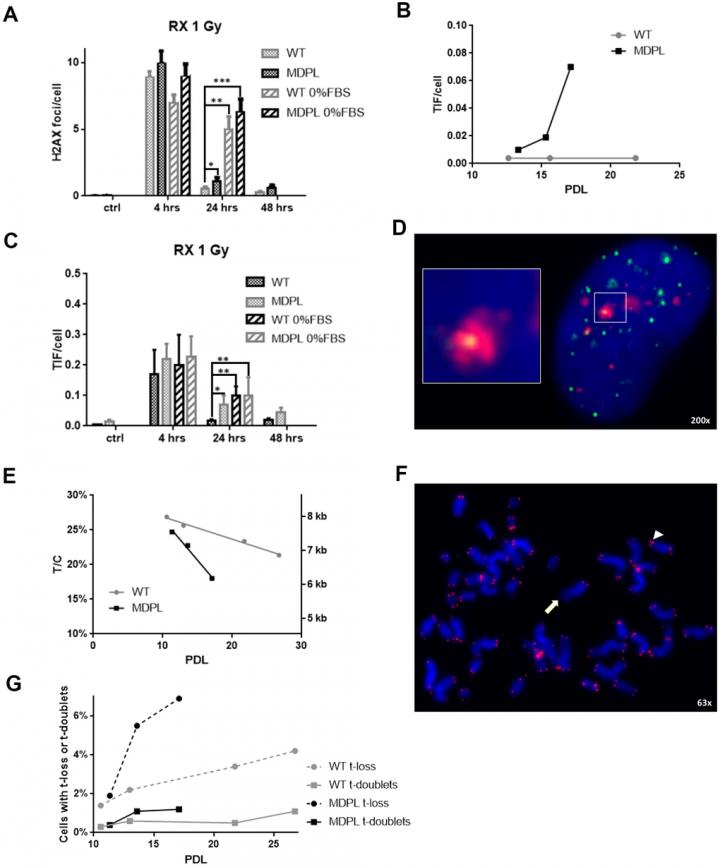
Aging-US published “Functional analysis of POLD1 p.ser605del variant: the aging phenotype of MDPL syndrome is associated with an impaired DNA repair capacity” which reported that Mandibular hypoplasia, Deafness and Progeroid features with concomitant Lipodystrophy define a rare systemic disorder, named MDPL Syndrome, due to almost always a de novo variant in POLD1 gene, encoding the DNA polymerase δ.
A decline of cell growth, cellular senescence and a blockage of proliferation in G0/G1 phase complete the aged cellular picture.
Moreover, the rate of telomere shortening was greater in pathological cells, suggesting the telomere dysfunction as an emerging key feature in MDPL.
These http://www.
Aging-US. results suggest an alteration in DNA replication/repair function of POLD1 as a primary pathogenetic cause of MDPL.com
The understanding of the mechanisms linking these cellular characteristics to the accelerated aging and to the wide spectrum of affected tissues and clinical symptoms in the MDPL patients may provide opportunities to develop therapeutic treatments for progeroid syndromes.
Dr. Maria Rosaria D’Apice The Tor Vergata Hospital and Dr. Antonella Sgura from “Roma Tre” University said, “Mandibular hypoplasia, Deafness and Progeroid features with concomitant Lipodystrophy, represent a rare systemic disorder, named MDPL syndrome (MDPL; OMIM #615381)”
MDPL was described for the first time in 2010, reporting seven subjects showing a clinical phenotype overlapping with mandibuloacral dysplasia syndromes such as mandibular hypoplasia, prominent eyes, stiff joints, beaked nose, and lipodystrophy, but also specific additional clinical hallmarks, including sensorineural hearing loss, hypogonadism and absent clavicular hypoplasia/acroosteolyses.
MAD and MDPL belong to the group of diseases characterized by premature aging, which can be caused by inheritable nuclear envelope and/or DNA repair defects.
Song and colleagues also demonstrated that POLD1 downregulation is able to block the cell cycle at G1 and G2/M phases and results in reduced DNA synthesis, demonstrating the potential role of POLD1 in the regulation of cell cycle progression.
Polδ serves to repair DNA lesions arising as a result of exposure to mutagens, acting in multiple forms of DNA repair, including nucleotide excision repair, double strand break repair, base excision repair, and mismatch repair.
In particular, these researchers shed light on the capacity of MDPL cells to respond and repair DNA induced-damage, especially at telomeric level.
The understanding of the pathogenic mechanism lying at the basis of the MDPL syndrome allows us to explore the link existing among DNA repair and age-related diseases.
The D’Apice/Sgura Research Team concluded in their Aging-US Research Output, “In order to respond to some of these intriguing questions, we are now generating human induced pluripotent stem cells from patient’s fibroblasts for applying genome-editing technologies, like the CRISPR-Cas9 nucleases, as tool for drug screening applications targeting DNA repair and development of therapeutic treatment.”
###
Sign up for free Altmetric alerts about this article
DOI – https:/
Full Text – https:/
Correspondence to: Maria Rosaria D’Apice email: [email protected] and Antonella Sgura email: [email protected]
Keywords: MDPL syndrome, POLD1 gene, age-related disease, DNA repair, telomere damage
About Aging-US
Launched in 2009, Aging-US publishes papers of general interest and biological significance in all fields of aging research as well as topics beyond traditional gerontology, including, but not limited to, cellular and molecular biology, human age-related diseases, pathology in model organisms, cancer, signal transduction pathways (e.g., p53, sirtuins, and PI-3K/AKT/mTOR among others), and approaches to modulating these signaling pathways.
To learn more about Aging-US, please visit http://www.
Aging-US is published by Impact Journals, LLC please visit http://www.
Media Contact
18009220957×105
[email protected]
Media Contact
Ryan James Jessup
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




