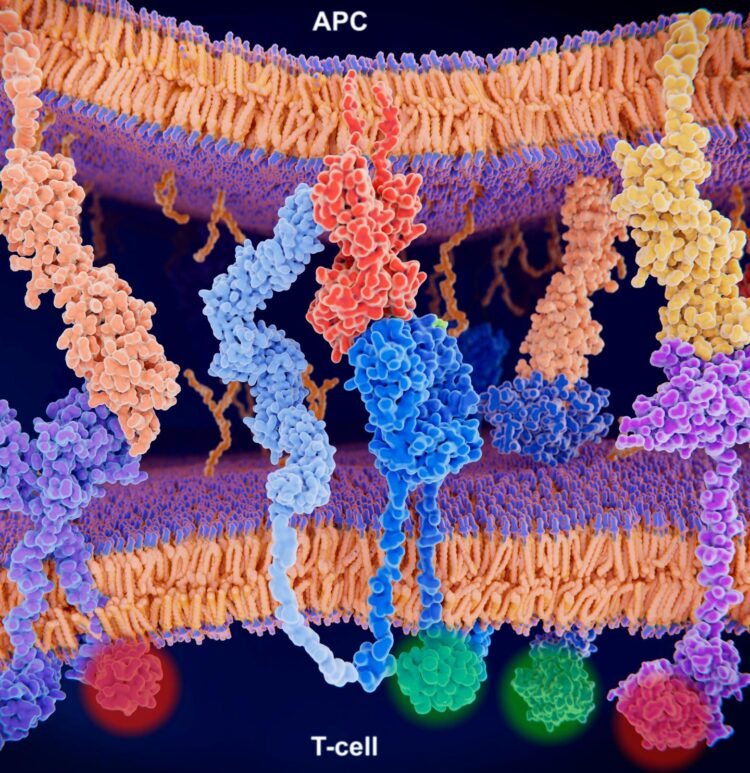
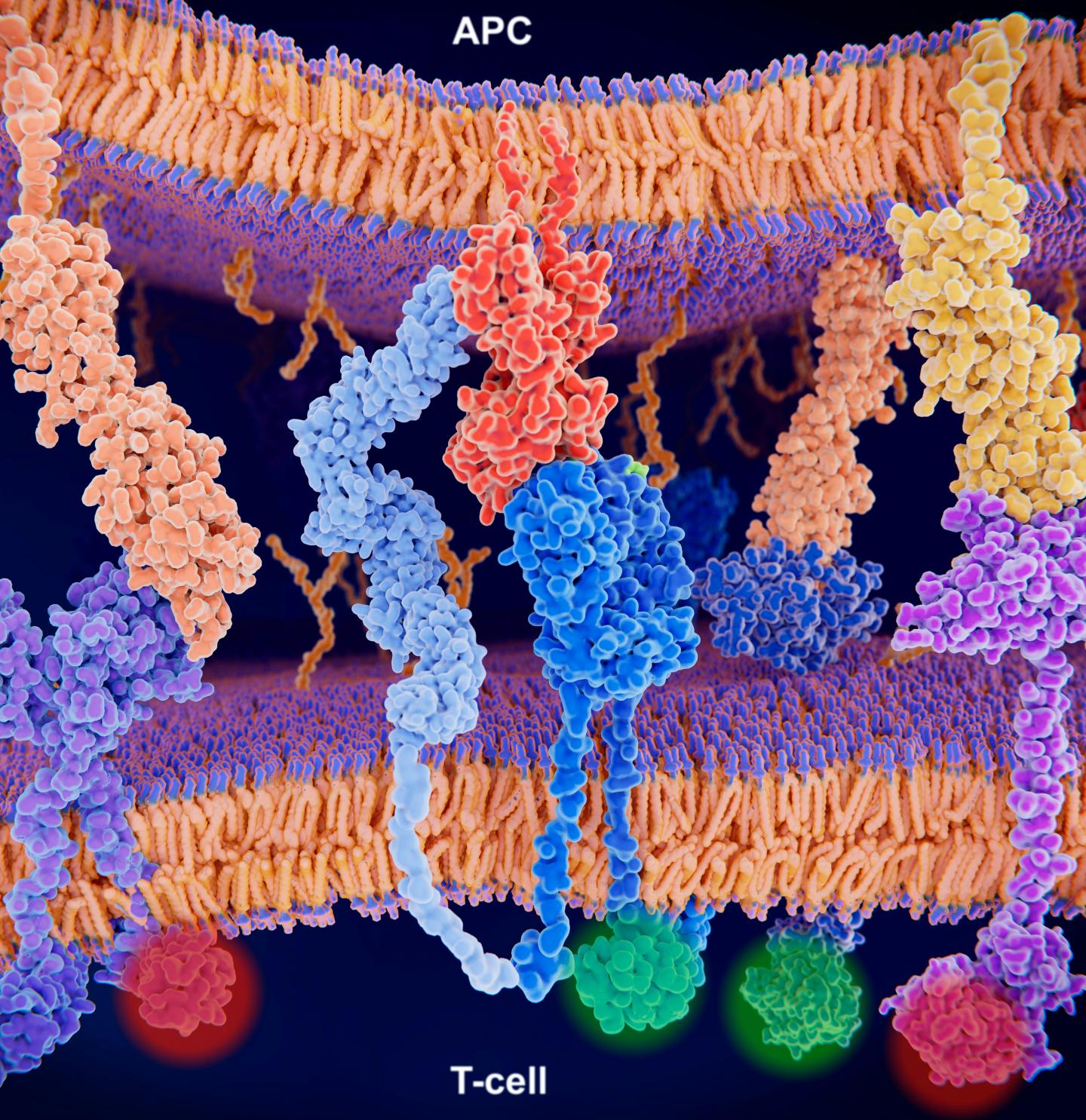
New statistical model analyzes the diversity of the T cell receptor repertoire that recognizes cancers, viruses, and bacteria
Credit: Art purchased from Alamy and modified by Dr. Tao Wang.
DALLAS – Jan. 6, 2020 – UT Southwestern scientists have developed a new method to study the molecular characteristics of T cells, critical immune cells that recognize and attack invaders in the body such as viruses, bacteria, and cancer.
The approach, described today in the journal Nature Methods, enables researchers to more easily analyze the roles of T cell receptors (TCRs) – the molecules on the surfaces of T cells that are responsible for recognizing pathogens.
“This could lead to a better understanding of how T cells work as well as new ways to harness T cells to fight disease,” says study leader Tao Wang, Ph.D., assistant professor of population and data sciences and a member of the Quantitative Biomedical Research Center at UTSW.
While some immune cells can simultaneously attack different pathogens, T cells are more targeted – every individual T cell has a distinct set of T cell receptors (TCRs) on its surface. Each receptor usually recognizes only one specific molecule, or “antigen.” One TCR, for instance, might bind only to a protein found in lung cancers, while a different TCR might bind just to an influenza virus. When a T cell encounters an antigen that binds to one of its TCRs, it becomes activated, prompting an immune response. To ward off the diverse set of potential invaders, humans have millions of different T cells in their bodies.
Scientists have attempted to study what makes different T cells and TCRs more or less effective, hampered so far by a lack of information about what various TCRs do. Generally, they assume that TCRs that look alike must bind to similar antigens, and that all TCRs activate T cells in a similar way.
To eliminate this guesswork, the research team developed a statistical model combining two existing technologies: TCR analysis, which measures a person’s TCR diversity, and single-cell RNA sequencing, which identifies the particular genes that are turned on or off in a T cell. Combining these technologies has been challenging since they both generate many thousands of pieces of data per experiment, and the data comes in two different forms. The new model, dubbed Tessa, uses powerful statistical methods to bridge this gap. Tessa reveals what happens to an individual T cell when its TCR recognizes an antigen, and in what way TCRs impact the function of the T cells. (Tessa stands for TCR functional landscape estimation supervised with scRNA-sequencing analysis.)
Using Tessa, the researchers studied 100,288 T cells from both healthy people and cancer patients. In cancer patients, they discovered that the variety of TCRs in T cells has a weaker influence on the functional status of T cells than on those found in healthy patients. This is likely because a plethora of other immune molecules, secreted into the tumor micro-environment, are influencing T cell activity in other ways. This observation – and others that are likely to result from more widespread use of Tessa – could have implications for scientists designing immune-based cancer treatments.
David Gerber, M.D., professor of internal medicine and population and data sciences, and associate director of clinical research in the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern, believes this work provides a completely new way of using single T cell sequencing data. “We envision deploying this promising tool to study the roles of T cells in immune-related adverse events caused by cancer immunotherapy through a recently funded NIAID U01 award,” he says.
“Previous techniques have involved a lot of guessing when it comes to the exact function of T cells and how T cell receptors associate with function,” adds Todd Aguilera, M.D., Ph.D., a UTSW assistant professor of radiation oncology, and an expert in immunotherapy, who is also collaborating with Wang. “I believe this method could help the identification of the most promising TCRs for personalized TCR-based immunotherapy and better define productive immune responses to guide identification of the optimal immunotherapy strategies.”
###
Other researchers on the study were Ze Zhang (first author), a UTSW graduate, Hongyu Liu, an exchange scholar at UTSW, and Danyi Xiong and Xinlei Wang of Southern Methodist University. This research was supported by funds from the National Institutes of Health (CCSG 5P30CA142543) and the Cancer Prevention and Research Institute of Texas (RP190208).
About UT Southwestern Medical Center
UT Southwestern, one of the premier academic medical centers in the nation, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty has received six Nobel Prizes, and includes 23 members of the National Academy of Sciences, 17 members of the National Academy of Medicine, and 13 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 2,500 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in about 80 specialties to more than 105,000 hospitalized patients, nearly 370,000 emergency room cases, and oversee approximately 3 million outpatient visits a year.
Media Contact
UT Southwestern Medical Center
[email protected]