Breakthrough findings from a new study point toward the first targeted therapy for an aggressive form of breast cancer that disproportionately affects black women
Credit: Gu Xiao
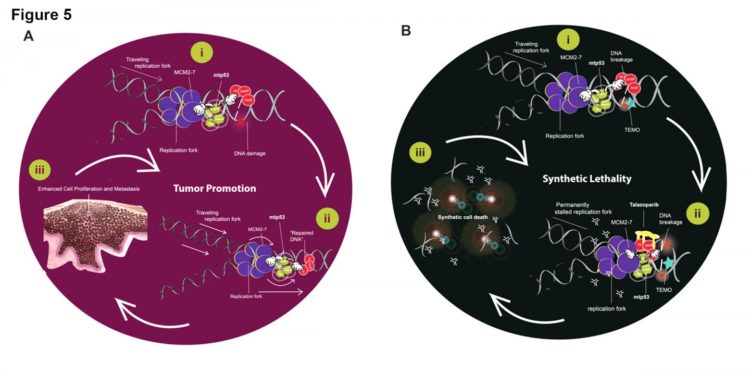
- Researchers identified two proteins — mutant tumor protein 53 (mtp53) and poly-ADP-Polymerase (PARP) — that are present and interacting with DNA during the replication process in patients with triple negative breast cancer.
- Suppressing these proteins through a combination of existing PARP-inhibiting therapies could be an effective way to interrupt the development of triple negative breast cancer cells and offer the first targeted treatment for this form of the disease.
- The findings may also reclassify triple negative breast cancer as a different form of the disease that can be treated by combining already existing therapies.
NEW YORK, January 29, 2020 (print edition) – A newly published study in the journal Cancer Research (appearing in print on February 1) signals a potential treatment breakthrough for patients with triple negative breast cancer — a form of the disease that disproportionately affects and also tends to develop more aggressively in black women. The paper, authored by researchers at The Graduate Center of The City University of New York and Hunter College in collaboration with scientists at Memorial Sloan Kettering Cancer Center and the University of Chicago, details findings about the interaction between two specific proteins during DNA replication that appears to drive growth of malignant cells in patients with triple negative breast cancer. The research team found the presence of mtp53 and PARP proteins in a large majority of patients with triple negative breast cancer. Their work suggests the proteins’ association and function, and suppressing their interactions could provide a possible target for stopping tumor growth.
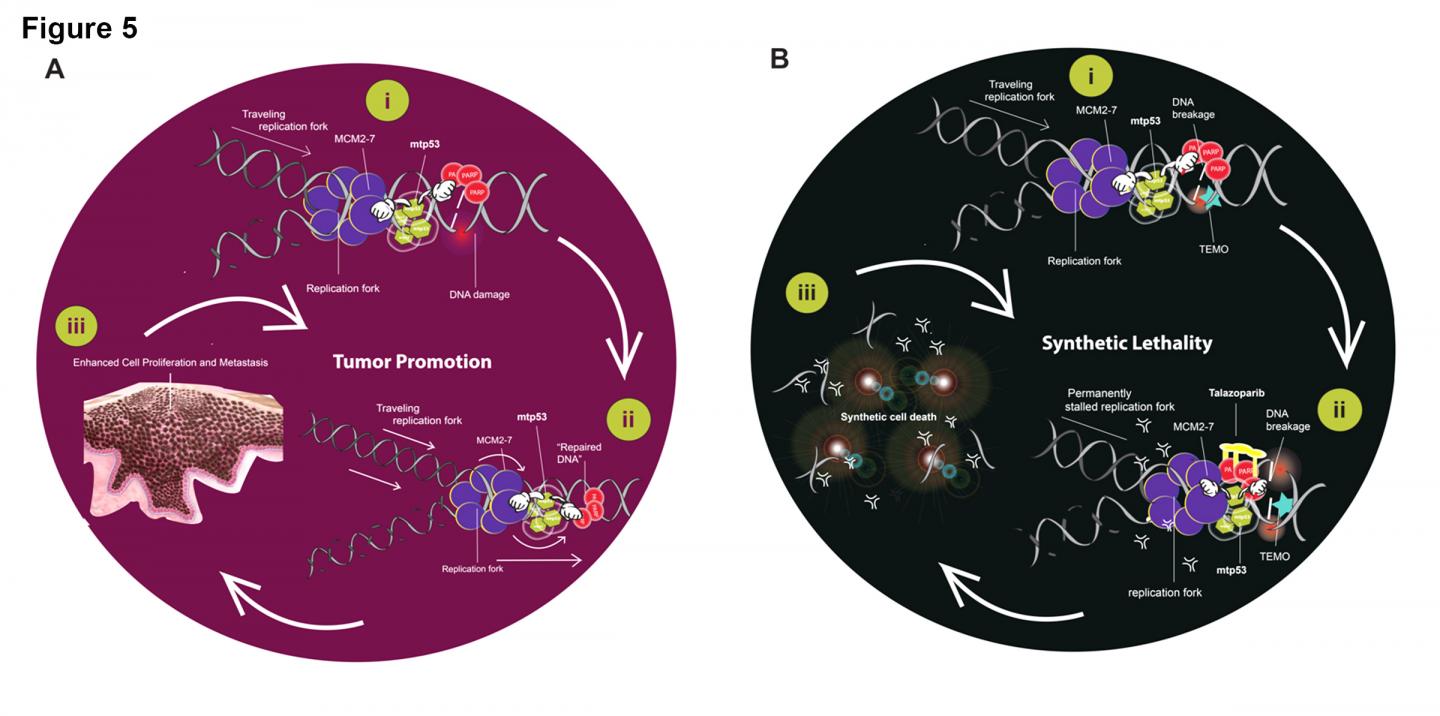
“Our new findings suggest that the presence of both mtp53 and PARP could serve as a good identifier of breast cancers that would respond to combined treatment with talazoparib — a PARP inhibitor that was developed to treat breast cancers with the BRCA mutation — and temozolomide — a chemotherapy agent that is used to treat some brain cancers,” said Graduate Center and Hunter College Professor Jill Bargonetti, whose lab conducted the research. “This is an exciting finding because it could lead to the first targeted therapy for triple negative breast cancer, enabling more precise and effective treatment of a very aggressive form of the disease.”
Researchers investigated a variety of breast cancer cell lines, patient-derived xenographs, tissue microarray samples, and data from The Cancer Genome Atlas to tease out the association and interaction between the mtp53 and PARP proteins in triple negative breast cancer. They discovered that high levels of these proteins are present on replicating DNA in these types of tumors, suggesting that the unusual presence and levels of these proteins may drive tumor growth. Review of the therapeutic mechanism of existing cancer drugs led the research team to identify talazoparib and temozolomide as existing drugs that could potentially be combined to create a targeted therapy that can effectively suppress the growth of triple negative breast cancer tumors.
“Our findings that mutant p53 and PARP participate in the DNA replication pathway will provide mechanism-derived dual biomarkers that aid in the diagnosis and treatment of these therapeutically elusive subsets of breast cancer,” said Gu Xiao, a research associate with Bargonetti’s lab and the paper’s first author.
The team’s next step would be to test whether the combined drugs successfully block replication of triple negative breast cancer cells in tumor samples in animal models. If the therapy ultimately proves successful it could lead to the reclassification of the disease to a category of cancers called mutant p53/PARP1 positive cancers, which are treatable by combination PARP inhibitor therapies.
###
This study was funded by The Breast Cancer Research Foundation.
About The Graduate Center of The City University of New York
The Graduate Center of The City University of New York (CUNY) is a leader in public graduate education devoted to enhancing the public good through pioneering research, serious learning, and reasoned debate. The Graduate Center offers ambitious students more than 40 doctoral and master’s programs of the highest caliber, taught by top faculty from throughout CUNY — the nation’s largest public urban university. Through its nearly 40 centers, institutes, and initiatives, including its Advanced Science Research Center (ASRC), The Graduate Center influences public policy and discourse and shapes innovation. The Graduate Center’s extensive public programs make it a home for culture and conversation.
Media Contact
Shawn Rhea
[email protected]
212-817-7180
Related Journal Article
http://dx.