Credit: Jennifer McCann/Penn State
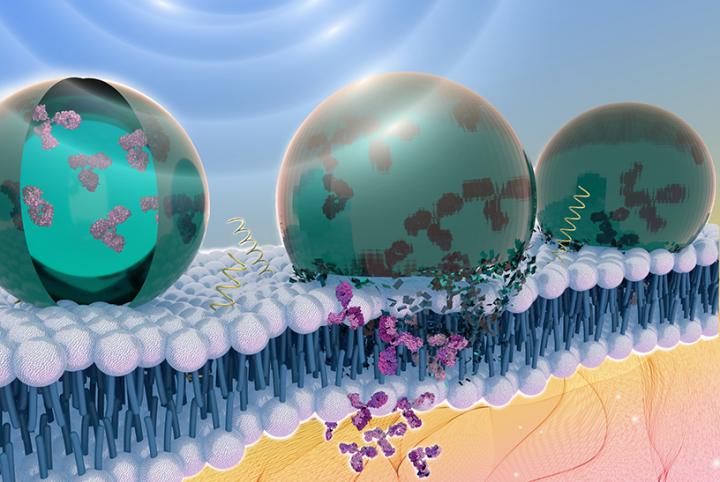
A new way to deliver therapeutic proteins inside the body uses an acoustically sensitive carrier to encapsulate the proteins and ultrasound to image and guide the package to the exact location required, according to Penn State researchers. Ultrasound then breaks the capsule, allowing the protein to enter the cell.
“When you expose the particle to ultrasound it opens a hole in the cell membrane that lasts for a couple of microseconds,” said Scott Medina, assistant professor of biomedical engineering, Penn State. “We can use this temporary opening to deliver antibodies, which are attractive therapeutic molecules in precision medicine that cannot otherwise get inside cells.”
These antibodies are emerging therapeutics for cancers, infectious diseases and rheumatoid arthritis, he said.
But getting the protein inside the nanoparticle carrier was not easy, which is why other researchers have had to resort to complicated and often poorly performing methods, such as attaching the cargo to the exterior of nanoparticles, resulting in inefficient protein release and off-target delivery.
The challenge with the new method was that the protein did not want to interact with the interior of the particle, which is made of a fluorous liquid, similar to liquid Teflon. Medina’s doctoral student, Janna Sloand, came up with a creative work around — a fluorous mask. These chemical masks have a counterbalance of polarity and fluorine content that allows the protein to interact with the fluorous liquid medium while maintaining the protein’s folded state and bioactivity.
“We had a lot of challenges developing this new method,” said Sloand, first author on the paper published recently in ACS Nano. “The most difficult was figuring out what kind of chemicals could mask the protein. That was definitely my eureka moment when I saw that it worked.”
In future work, the team will explore the use of their ultrasound-programmable material as a platform for image-guided delivery of therapeutic proteins and gene editing tools.
In related therapeutic applications, they are leveraging this technology to deliver antibodies that can alter abnormal signaling pathways in tumor cells to effectively ‘turn-off’ their malignant traits. In other work they are delivering gene editing tools, like CRISPR constructs, to enable ultrasound-controlled genome engineering of cells in complex 3D tissue microenvironments.
Importantly, these delivery applications can all be performed using ultrasound techniques already employed in hospitals, which they hope will enable the rapid translation of this technology for precision healthcare.
###
Additional authors on the paper, titled “Ultrasound-Guided Cytosolic Protein Delivery via Transient Fluorous Masks,” are doctoral student Teddy T Nguyen, master’s student Scott A Zinck, post-doctoral scholar Erik C Cook, Assistant Research Professor Tawanda J Zimudzi, Professor Scott A. Showalter, Professor Adam B. Glick, and Assistant Professor Julianna C Simon.
The National Science Foundation funded this research.
Media Contact
A’ndrea Elyse Messer
[email protected]
Related Journal Article
http://dx.




