Researchers from Tokyo Medical and Dental University (TMDU) identify lipids stimulating self-repair mechanisms in the brain after ischemic stroke
Credit: Department of Neuroinflammation and Repair, TMDU
Researchers from Tokyo Medical and Dental University (TMDU) identify lipids stimulating self-repair mechanisms in the brain after ischemic stroke
Tokyo, Japan – Patients often experience functional decline after an ischemic stroke, especially due to the brain’s resistance to regenerate after damage. Yet, there is still potential for recovery as surviving neurons can activate repair mechanisms to limit and even reverse the damage caused by the stroke. How is it triggered though?
In a study published recently in Neuron, researchers from Tokyo Medical and Dental University (TMDU) provided new insights regarding this question by identifying a new mechanism. They discovered that neurons surrounding the area of cell death secrete lipids that can trigger brain-autonomous neural repair after ischemic brain injury.
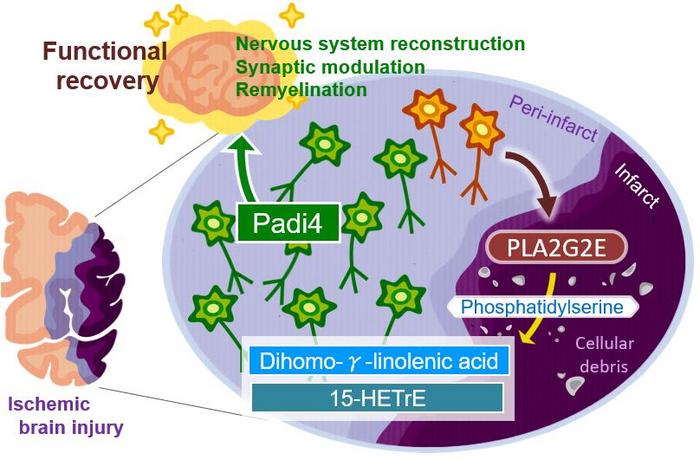
An ischemic stroke occurs when the blood supply to the brain is blocked and results in the death of brain cells. This condition is life-threatening, and patients will likely develop functional disabilities. Although the adult brain can self-repair, the underlying mechanisms need further clarification.
Inflammation of the brain contributes to the effects of ischemic stroke. “There is evidence that more lipids are produced after tissue injuries and contribute to regulating inflammation,” says Takashi Shichita, senior author of the study. “We investigated the changes in lipid metabolite production in mice after ischemic stroke. Interestingly, the levels of a specific fatty acid called dihomo-γ-linolenic acid (DGLA) and its derivatives increased after the stroke.”
The researchers further discovered that a protein known as PLA2GE2 (Phospholipase A2 Group IIE, an enzyme) mediates DGLA increase. By manipulating the expression of PLA2GE2, they also showed its impact on functional recovery. Deficiency of PLA2GE2 led to more inflammation, lower expression of factors stimulating neuronal repair, and more tissue loss. The team carried on with identifying the targets of PLA2GE2/DGLA.
“When we look at genes expressed in mice lacking PLA2GE2, we found low levels of a protein called peptidyl arginine deiminase 4 (PADI4),” explains Akari Nakamura, lead author of the study. “PADI4 regulates transcription and inflammation. Remarkably, expressing PADI4 in mice limited the extent of tissue damage and inflammation after ischemic stroke!” Additionally, the study shows that PADI4 promotes the transcription of genes involved in brain repair. It also identifies the whole signaling pathway involved in this process.
Most data were obtained in a mouse model of ischemic stroke. Yet, the recovery pathway likely exists in humans as the researchers found that neurons surrounding the stroke site express PLA2G2E and PADI4 in humans. Moreover, another recent study reported that the lower serum DGLA level was correlated with the severe ischemic stroke and cognitive disorders in humans.
This study describes a new mechanism that triggers brain repair after an ischemic stroke, which might lead to the development of compounds promoting PADI4’s effects, that stimulate the recovery of patients. It could also change our current understanding and approach toward Eicosapentaenoic acid (EPA) or Docosahexaenoic acid (DHA), as the only beneficial lipids for preventing atherosclerosis and vascular diseases.
###
The article, “PLA2G2E-mediated lipid metabolism triggers brain-autonomous neural repair after ischemic stroke,” was published in Neuron at DOI: 10.1016/j.neuron.2023.06.024
Journal
Neuron
DOI
10.1016/j.neuron.2023.06.024
Article Title
PLA2G2E-mediated lipid metabolism triggers brain-autonomous neural repair after ischemic stroke




