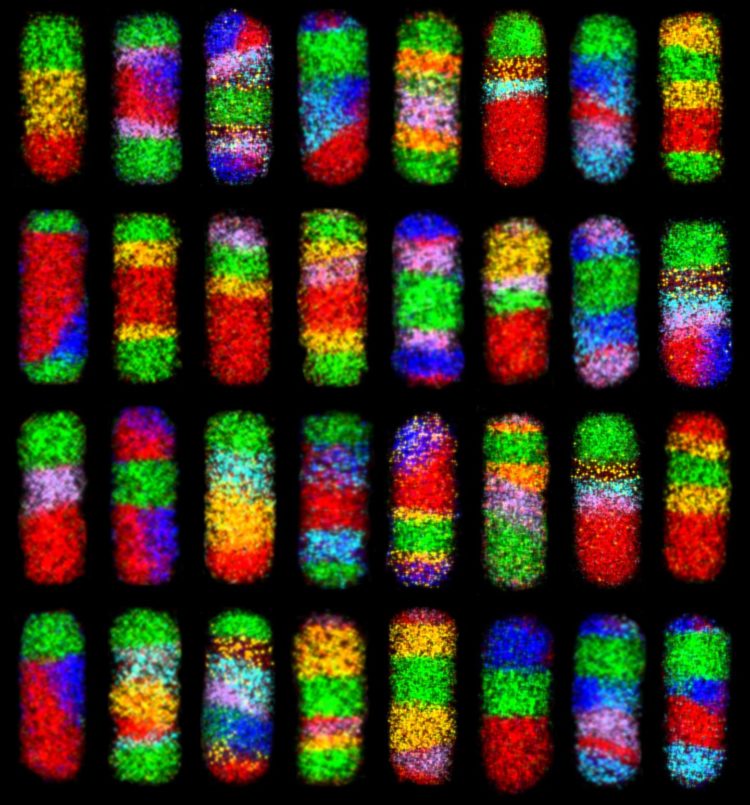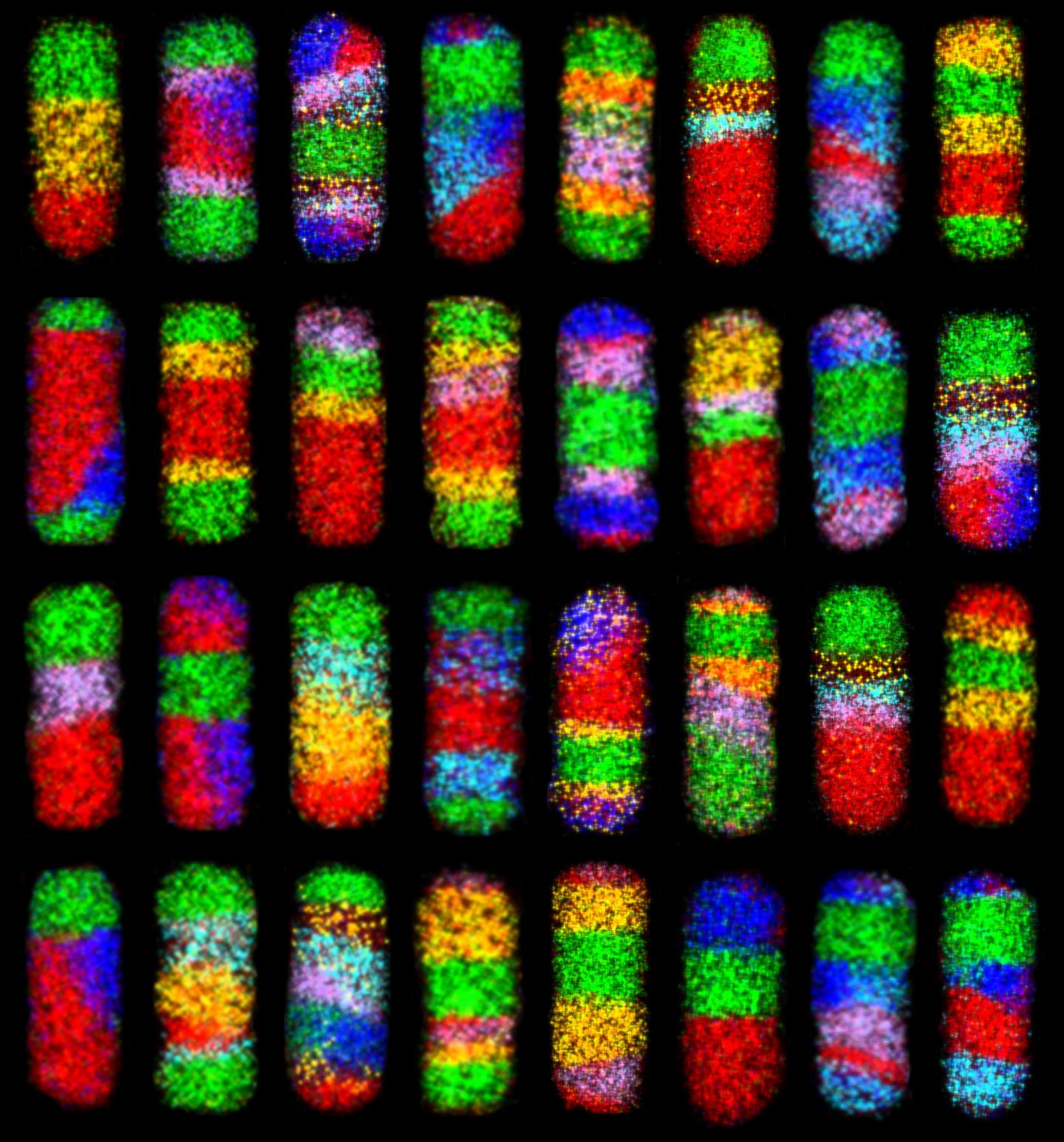
Credit: Schaak Laboratory, Penn State
Using straightforward chemistry and a mix-and-match, modular strategy, researchers have developed a simple approach that could produce over 65,000 different types of complex nanoparticles, each containing up to six different materials and eight segments, with interfaces that could be exploited in electrical or optical applications. These rod-shaped nanoparticles are about 55 nanometers long and 20 nanometers wide–by comparison a human hair is about 100,000 nanometers thick–and many are considered to be among the most complex ever made.
A paper describing the research, by a team of Penn State chemists, appears January 24, 2020 in the journal Science.
“There is a lot of interest in the world of nanoscience in making nanoparticles that combine several different materials–semiconductors, catalysts, magnets, electronic materials,” said Raymond E. Schaak, DuPont Professor of Materials Chemistry at Penn State and the leader of the research team. “You can think about having different semiconductors linked together to control how electrons move through a material, or arranging materials in different ways to modify their optical, catalytic, or magnetic properties. We can use computers and chemical knowledge to predict a lot of this, but the bottleneck has been in actually making the particles, especially at a large-enough scale so that you can actually use them.”
The team starts with simple nanorods composed of copper and sulfur. They then sequentially replace some of the copper with other metals using a process called “cation exchange.” By altering the reaction conditions, they can control where in the nanorod the copper is replaced–at one end of the rod, at both ends simultaneously, or in the middle. They can then repeat the process with other metals, which can also be placed at precise locations within the nanorods. By performing up to seven sequential reactions with several different metals, they can create a veritable rainbow of particles–over 65,000 different combinations of metal sulfide materials are possible.
“The real beauty of our method is its simplicity,” said Benjamin C. Steimle, a graduate student at Penn State and the first author of the paper. “It used to take months or years to make even one type of nanoparticle that contains several different materials. Two years ago we were really excited that we could make 47 different metal sulfide nanoparticles using an earlier version of this approach. Now that we’ve made some significant new advances and learned more about these systems, we can go way beyond what anyone has been able to do before. We are now able to produce nanoparticles with previously unimaginable complexity simply by controlling temperature and concentration, all using standard laboratory glassware and principles covered in an Introductory Chemistry course.”
“The other really exciting aspect of this work is that it is rational and scalable,” said Schaak. “Because we understand how everything works, we can identify a highly complex nanoparticle, plan out a way to make it, and then go into the laboratory and actually make it quite easily. And, these particles can be made in quantities that are useful. In principle, we can now make what we want and as much as we want. There are still limitations, of course–we can’t wait until we are able to do this with even more types of materials–but even with what we have now, it changes how we think about what is possible to make.”
###
In addition to Schaak and Steimle, the research team at Penn State included Julie L. Fenton. The research was funded by the U.S. National Science Foundation.
Media Contact
Sam Sholtis
[email protected]
814-865-1390