Researchers from Kanazawa University purified the pore-forming bacterial toxin Monalysin to study how the innate immune system fights off bacterial toxins
Credit: Kanazawa University
Kanazawa, Japan – Although the innate immune system is the front line of defense against microbial infections, the complex mechanisms of innate immunity are incompletely understood. In a new study, researchers from Kanazawa University synthesized and characterized the bacterial toxin Monalysin to enable the study of how the innate immune system and toxin-producing bacteria interact with each other.
The innate immune system detects microbial infections through sensing either microbial molecules (pathogen-associated molecular patterns, or PAMPs) or host signaling molecules that are released from damaged host cells (damage-associated molecular patterns, or DAMPs). The bacterium Pseudomonas entomophila has been utilized as a tool to study the mechanisms of DAMPs in the gut. P. entomophila infects insects and damages intestinal cells using a pore-forming toxin called Monalysin. Monalysin is secreted as an inactive pro-toxin, which is then activated by certain proteins called proteases. Although the fruit fly, Drosophila, protects itself from activation of the pro-toxin by building a physical barrier against proteases, it can still take damage upon exposure to the toxin.
“Activated Monalysin forms pores in the plasma membrane of host cells, resulting in cell death, so it is important for the host to prevent its activation,” says corresponding author of the study Takayuki Kuraishi. “We wanted to purify and functionally characterize Monalysin from P. entomophila to develop a tool that could help us understand how the host and bacteria that produce pore-forming toxins interact.”
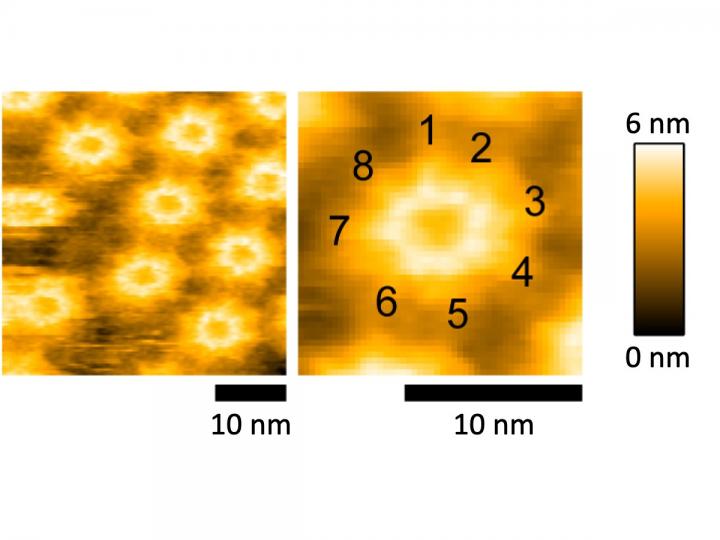
To achieve their goal, the researchers cultured P. entomophila and purified pro-Monalysin from their lysates. By reacting the purified toxin with Drosophila cells, the researchers confirmed its toxic effect when cell viability dropped significantly as more pro-Monalysin was added to the cells. To confirm that purified Monalysin forms pores, the researchers added activated Monalysin onto a chip covered with a lipid bilayer, similar to the plasma membrane of cells. By measuring the electrical current resulting from ion passage through the formed pores, the researchers showed that Monalysin forms pores around 0.7-1nm in diameter. To analyze the structural composition of Monalysin, the researchers then turned to atomic force microscopy (AFM), which provides high-resolution images by touching the surface with a sensitive mechanical probe. Using AFM, the researchers showed that eight Monalysin molecules came together to form pores in the plasma membrane. By combining AFM with high-speed imaging, the researchers then demonstrated that activated Monalysin preferentially inserted into the edge of the plasma membrane, suggesting that highly curved parts of membranes are the sites of their action.
“These are striking results that show how Monalysin functions at the molecular level,” says Kuraishi. “Our findings could help understand how the innate immune system fights off bacteria that produce pore-forming toxins.”
###
Media Contact
Tomoya Sato
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




