Issued in conjunction with the Michael J. Fox Foundation, important consensus guidelines for running proof of concept preclinical and clinical trials of drugs targeting alpha-synuclein to slow or arrest the progression of the disease
Credit: Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, Ill.
Amsterdam, NL, December 20, 2018 – A recently discovered protein, alpha-synuclein, has become one of the most attractive targets for developing new drugs with the potential to slow down or arrest the progression of Parkinson’s disease (PD). Experts in the field of Parkinson’s research have now proposed a roadmap for preclinical and clinical trials investigating compounds targeting alpha-synuclein. Their consensus white paper is published in the Journal of Parkinson’s Disease.
Alpha-synuclein is of key interest to PD researchers because it is a major constituent of Lewy bodies, protein clumps that are the pathological hallmark of PD, and mutations in the gene that encodes alpha-synuclein cause PD. Not surprisingly, intensive efforts have been underway to study the normal and pathological role of alpha-synuclein as well as its potential as a target for neuroprotective therapies. There are at least five alpha-synuclein–targeted therapeutics currently under investigation. These have the potential to slow or arrest the progression of PD and other synucleinopathies, such as Dementia with Lewy Bodies, Multiple System Atrophy and Pure Autonomic Failure.
“With alpha-synuclein undoubtedly playing some role in PD pathogenesis, and there being such a diverse portfolio of experimental therapies that target the protein, one can be optimistic and hope that one of the approaches will eventually be successful in slowing disease progression,” says Patrik Brundin, MD, PhD, Associate Director of Research, Professor and Director of the Center for Neurodegenerative Science, Van Andel Research Institute, Grand Rapids, MI, and Co-Editor-in-Chief of the Journal of Parkinson’s Disease.
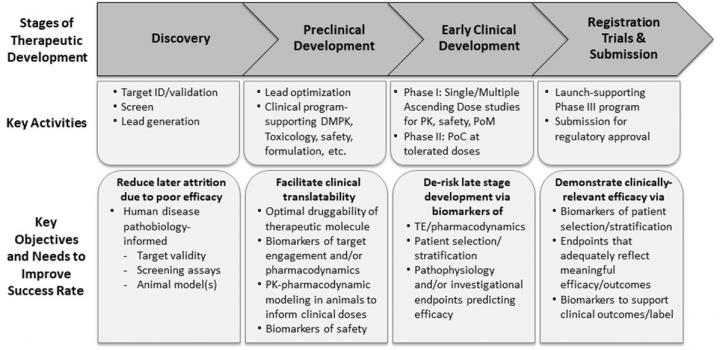
In 2017, The Michael J. Fox Foundation convened the Alpha Synuclein Clinical Path Working Group comprised of PD research leaders from across academia and industry. This group was tasked to develop a strategic consensus and make recommendations in preclinical and clinical research directed at alpha-synuclein–targeted therapies for PD.
In this consensus white paper, experts provide a translational framework of de-risking the development of alpha-synuclein–targeted therapies. Specifically, the paper discusses the use of fit-for purpose animal models, biomarkers that inform clinical trial design, such as doses and dosing regimen, as well as patient enrichment strategies. Finally, the authors discuss considerations for the design of clinical proof of concept trials that integrate not only pathophysiologic endpoints, but also the emerging technology of wearable devices to monitor clinical outcomes.
“Multiple therapeutics have recently entered clinical trials, and critical human data that will inform all alpha-synuclein–based therapeutic development programs are on the horizon,” says lead author Kalpana M. Merchant, PhD, of Vincere Biosciences, Inc. and the Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL. “Although these efforts face many profound challenges, including the lack of key tools such as an alpha-synuclein–based imaging agent and the inherent difficulty of demonstrating clinical efficacy in slowly progressive neurodegenerative diseases, we remain optimistic that meaningful strides toward the ultimate identification and approval of alpha-synuclein–based disease-modifying therapeutics will be made in the near future.”
PD is the second most common neurodegenerative disorder, affecting approximately 1.2 percent of the world population over the age of 70. Although several new therapies that address motor or non-motor symptoms of PD have been approved, none of these are able to slow disease progression. In the US alone, an estimated 630,000 people had PD in 2010. With anticipated demographic changes due to an aging population, and if no disease-modifying treatment is found, the prevalence is expected to reach 930,000 by 2020 and 1.24 million 40 by 2030.
###
Media Contact
Diana Murray
[email protected]
718-640-5678
Related Journal Article
http://dx.




