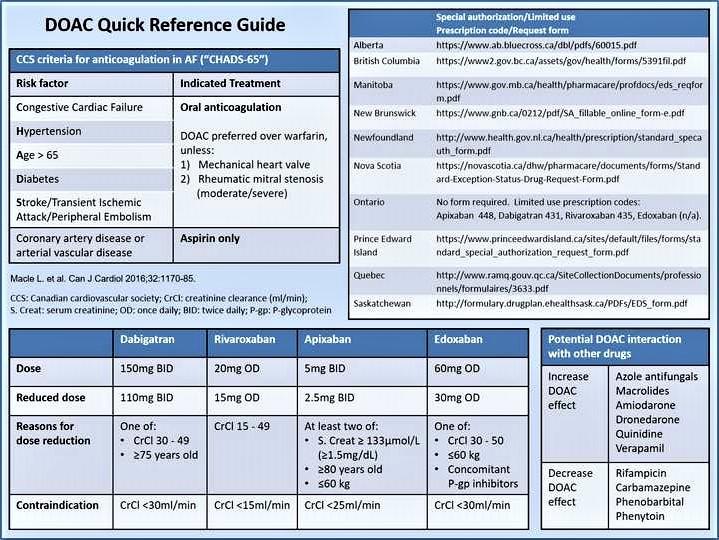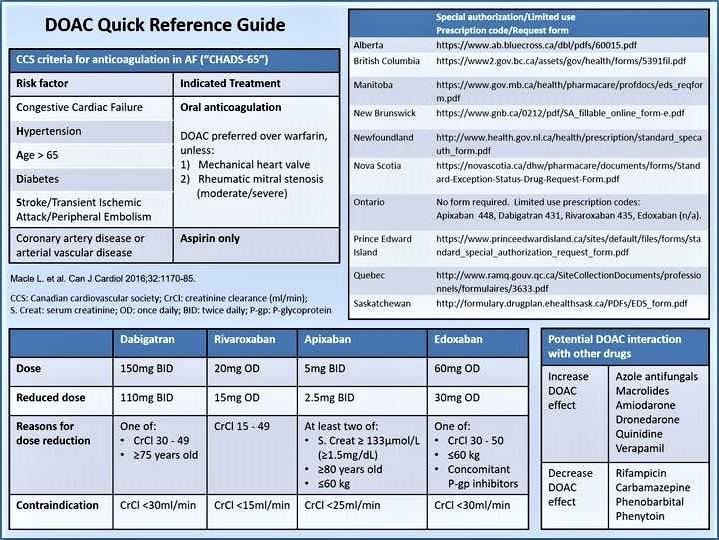
Credit: Canadian Journal of Cardiology
Philadelphia, November 5, 2018 – International guidelines recommend direct oral anticoagulants (DOACs) over warfarin to prevent stroke for most patients with atrial fibrillation (AF). However, a substantial portion of patients in Canada, who would benefit from anticoagulation, do not receive it adequately or at all. Experts review the evidence for the use of DOACs in the Canadian Journal of Cardiology, discuss reasons for the large gap between guidelines and clinical practice, including policy and funding barriers, and propose strategies for the future.
AF affects about 350,000 patients in Canada and about 10 times as many in the United States. AF is the most common heart rhythm disorder in the world with major impact on public health, especially due to increased risk of stroke. Stroke due to AF still comprises up to 15 percent of all stroke cases, and disabling stroke remains disproportionately more common in patients with AF than in patients without. Although anticoagulation drug therapy is an effective treatment, its use in Canada is slow to reflect evidence-based guidelines.
"Despite the evidence and near unanimity of clinical practice guidelines, these drugs go unprescribed for many, perhaps even most, eligible patients," noted lead author Chris S. Simpson, MD, of the Queen's University-Kingston Health Science Center, Kingston, ON, Canada. "The result is that many preventable strokes are still happening every day in Canada."
DOAC indications for stroke risk reduction were initially incorporated into the Canadian Cardiovascular Society (CCS) guidelines in 2010. This recommendation has subsequently been included in the guidelines of other major societies such as the European Society of Cardiology (ESC) and the American Heart Association (AHA). The CCS then recommended a preference for dabigatran over warfarin because of dabigatran's greater efficacy and safety. The 2014 CCS guidelines strongly recommended with "high-quality evidence" that DOACs should be preferred over warfarin when an OAC is indicated for stroke prevention in AF but highlighted the lack of governmental approval of DOACs and saw the lack of reimbursement in Canada as a "major challenge" to following the recommendations.
The authors call attention to patient-physician factors in DOAC decision-making and identify barriers to using DOACs in Canada:
- Perceived cost is a major, if not the main barrier to prescribing DOACs.
- High initial cost due to temporal gap between guidelines recommendation and funding approval.
- Antiquated perception among physicians that aspirin is a suitable alternative to DOAC, lack of experience in managing DOAC, and fear of bleeding complications.
- Mandatory trial with warfarin in some Canadian provinces.
- Lack of experience and limited availability of specific reversal agents to manage bleeding.
- Lack of patient awareness of stroke risk in AF and information regarding DOAC usage and access.
- Concurrent release of multiple DOACs and patient-targeted information on DOAC profiles and dosing schedules may delay patient-physician decision to start DOAC.
- Limited use coverage that denies patients who are eligible based on guidelines, forcing suboptimal therapy or paying out-of-pocket if not covered by private insurance.
- Physicians required to justify clinical decision in providing evidence-based guideline-recommended treatment.
- Physicians' lack of guideline awareness contributing to reduced confidence in starting DOAC therapy. Guideline awareness may be part of the reason why cardiologists (more than other specialists) are more likely to start DOAC treatment.
Recognition of this "guidelines-policy gap" is the first step, say the authors, in developing a strategy to improve implementation of DOAC usage in Canada, including:
- Improving awareness of the risk and preventability of stroke.
- Enabling physicians to provide evidence-based DOAC usage.
- Empowering patients to improve adherence and persistence.
- Collecting real-life data that encourage patient self-monitoring and physician outcomes auditing, and build evidence that is useful for policy makers.
- Using post-marketing data in negotiating shared risk-management between pharmaceuticals and government to improve access to DOACs.
"This discussion highlights a significant conflict between clinical practice guidelines processes and governmental regulatory and approval processes," commented Dr. Simpson. "Both processes aim to act in the best interests of patients and the public and should therefore work in concert rather than in conflict. The result of this conflict is a delay in the uptake of a beneficial therapy which saves lives and prevents significant morbidity."
Observations of other government-funded universal healthcare systems may be helpful in providing solutions to managing the policy and funding barriers in Canada. The authors point out that the National Health Service (NHS) in the UK is considered to be a good peer comparator to the Canadian health system. The NHS bases its practice on ESC guidelines and its funding policy is guided by the National Institute for Health and Clinical Excellence (NICE). NICE guidelines emphasize individualized assessment and discussion with patients regarding benefits and risks of each OAC option and recommends against a "trial of warfarin" because this would prolong the time to find the best therapy.
The UK also employs several strategies to increase access to guideline-recommended therapies that are awaiting approval or have insufficient funding, such as the Patient Access Scheme, which allows expensive, non-approved therapies to be more accessible through discount negotiations between manufacturer and the Department of Health. Similar schemes in Australia, New Zealand, and Asia have also improved access.
"Every doctor wants their patient to have the best treatment possible. Every provincial government has a duty to their citizens and taxpayers to spend public money in a way that provides the best value. Only by working together can we hope to achieve both goals," Dr. Simpson concluded.
###
Media Contact
Eileen Leahy
[email protected]
732-238-3628
@elseviernews
http://www.elsevier.com
Related Journal Article
http://dx.doi.org/10.1016/j.cjca.2018.07.476