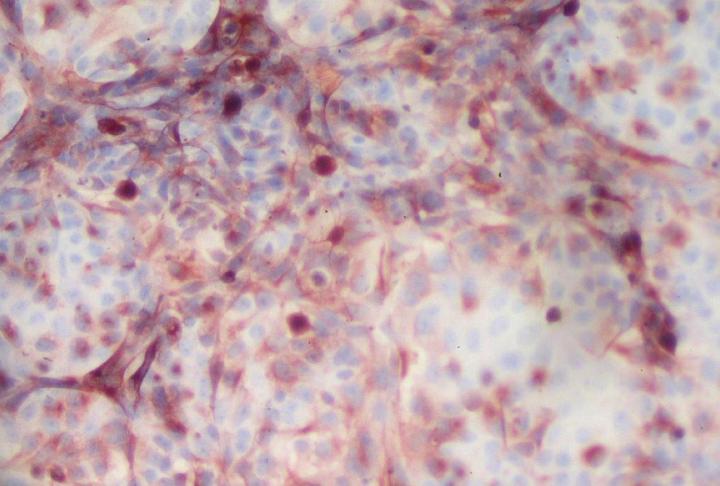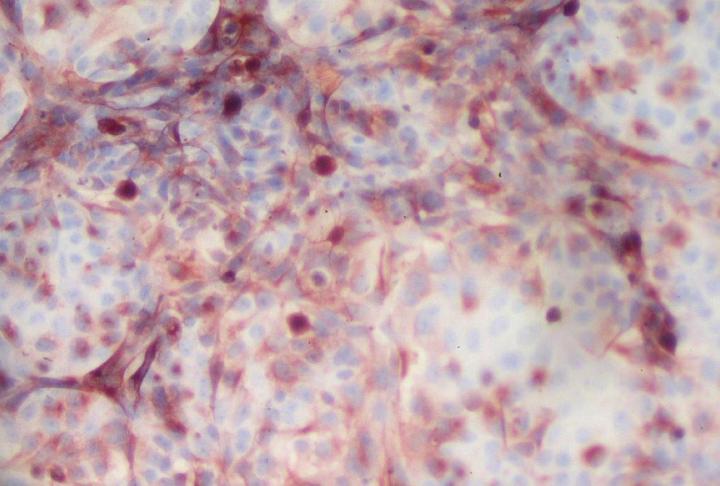
Credit: © Marie-Christine Cumont and Monique Lafon – Institut Pasteur
Scientists from the Institut Pasteur, the CNRS and Sanofi Pasteur have recently developed a novel alternative method to animal testing that can be used to verify the safety of vaccines such as the yellow fever vaccine. This original approach is based on the development of an in cellulo device using a 3D culture model, the "BBB-Minibrain", to evaluate the safety of live vaccines for human use. The model was developed by the Institut Pasteur and a patent application has been filed by the Institut Pasteur and Inserm. It raises hopes for a reduction in the use of animals in quality control, especially in the tests carried out by the pharmaceutical industry to meet the requirements of regulatory authorities. The results of this research were published in the journal Biologicals in May 2018, and online on March 24th.
For several years now, following the adoption of EU Directive 2010/63/EU,1 the scientific community has been actively seeking to reduce the practice of animal testing. But in many cases, these efforts are hindered by a lack of acceptable alternatives that satisfy regulatory authorities. This is particularly the case for the regulatory testing required for live viral vaccines, such as the yellow fever vaccine; suppliers must demonstrate that the seed lots used to produce vaccine batches sold on the market do not represent a risk of neurotoxicity. These tests are currently performed on animals, which are monitored for the emergence of any clinical signs in the central nervous system that may suggest neurotoxic side effects.
Against this backdrop, Institut Pasteur scientists developed a 3D culture model mimicking the human blood-brain interface, the "BBB-Minibrain", in 2014. This model, formed of a blood-brain barrier (BBB) associated with a mixed culture of neurons, astrocytes and microglia (a "minibrain"), can be used to detect when viruses enter the brain through the BBB, their multiplication in the minibrain and the emergence of any neurotoxic effects. A patent application (WO2016038123) was filed for the model.
The scientists set out to test the BBB-Minibrain's ability to pinpoint and amplify any rare mutant particles with neuroinvasive and neurovirulent properties that are found in seed lots for live viral vaccines. They chose to use two yellow fever virus vaccine strains, including the strain currently used to produce the vaccine, which does not cause neurotoxicity.
Working with Sanofi Pasteur research teams, they demonstrated that the BBB-Minibrain can be used to identify any rare viral particles in vaccine preparations that have acquired the ability to enter the brain and multiply there. This test therefore paves the way for the rejection of any seed lots containing mutant viruses capable of entering the brain and becoming neurovirulent.
As Monique Lafon, lead author of the study and Director of the Virology Department at the Institut Pasteur, explains, "replacing animal testing is a major challenge for research. The BBB-Minibrain model is an ingenious tool that will facilitate our analysis of the basis for neurovirulence in these viruses, which colonize the brain via the bloodstream."
These findings represent a first proof of concept and feasibility for the development of an alternative test that complies with the "3Rs" principle. Work to develop this test is ongoing. The long-term aim is to secure approval for the new test from regulatory authorities.
The BBB-Minibrain model raises hopes for the development of an alternative method that can be used by the pharmaceutical industry to perform regulatory tests on live viral vaccines. The aim of this method is to reduce the use of animals while ensuring strict monitoring of any scientific benefits and breakthroughs in the area of human health.
###
1 This Directive enshrines the 3Rs approach: the treatment and use of living animals for scientific purposes are governed by the principles of Replacement, Reduction and Refinement as established at international level.
Media Contact
Press Office
[email protected]
http://www.pasteur.fr
Original Source
https://www.pasteur.fr/en/press-area/press-documents/yellow-fever-new-method-testing-vaccine-safety http://dx.doi.org/10.1016/j.biologicals.2018.03.004