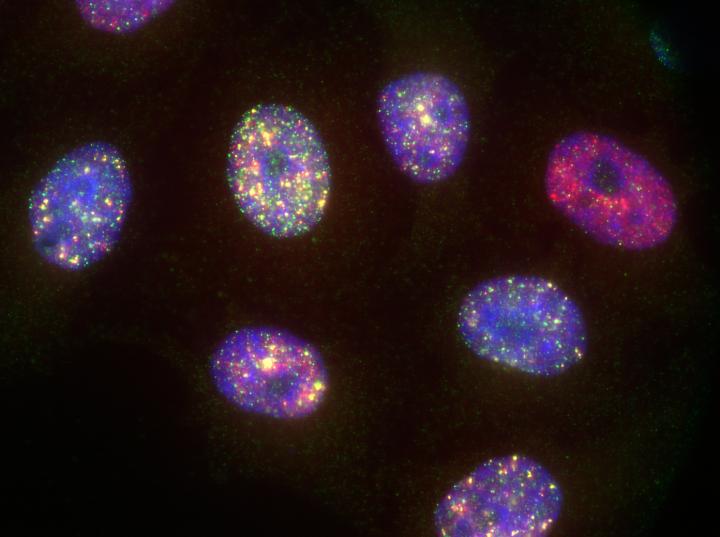
Credit: Michel Owusu / CeMM
When a cell develops into a tumor, something has gone terribly wrong: the uncontrolled growth, invasion of nearby tissues and finally metastasis are the result of many consecutive DNA mutations. Such an accumulation of demolished genetic material often derives from initial environmental exposures, enzymatic activities or defects in DNA replication or DNA repair mechanisms. Each of those initial mutagenic conditions creates their own pattern of DNA damage called mutational signature. Deciphering them could theoretically allow us to trace back the initial cause of a tumor, profile its properties and help find a therapeutic strategy.
However, reading those mutational signatures in tumor samples is a difficult task, as the large amount of mutations that a patient acquires during its lifetime create a noisy and uncontrolled system – even the best clinical data will, at most, provide only associations. Therefore, the group of Joanna Loizou, Principal Investigator at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, in collaboration with researchers from the Wellcome Trust Sanger Institute, developed an experimental setup to validate the concept of mutational signatures in cell culture.
In special human cell lines, optimized for genome editing, the researchers destroyed nine different genes – one gene per cell line – for DNA repair using CRISPR-Cas9 technology. Subsequently, they cultivated single cells from each of those nine experiments and allowed them to proliferate for one month. Eventually, the scientists analyzed the entirety of mutations that accumulated over this time period in the cell line deriving from the single progenitor that lacks a specific DNA repair gene. With specially developed computational methods, they were able to find the genomic scars that a missing DNA repair gene left in the genome of the affected cells – they were almost identical to mutational signatures found in cancer cells.
"Our results validate mutational signatures across all classes of mutations", Joanna Loizou summarizes the findings. "For the first time, this theoretical concept was confirmed in a highly controlled experimental setup". However, a single gene defect is not restricted to only one mutational signature, Loizou adds: "We found that some defects in DNA repair genes engender multiple mutational signatures of different classes". The converse is also true, as Michel Owusu, PhD Student in Loizou's laboratory and co-first author of the paper explains: "A mutational signature may not necessarily reflect a defect of one gene, as it could also arise through the malfunctioning of another related gene involved in the affected DNA repair mechanism".
The findings of this study not only confirm an analytical principle that describes mutational processes and cancer development, mutational signatures are a direct mechanistic read-out of specific dysfunctions of a cell. Thus, even if the underlying gene defect is unknown, mutational signatures could be used as biomarkers for the molecular characterization of tumors – a new diagnostic tool to improve the precise and personalized treatment of cancer.
###
Attached pictures: 1) Hap1 Cells stained for different DNA damages (yellow = antibody against phosphorilated ATM; red = antibody against 53BP1; a regulator of NHEJ; blue = DAPI = DNA) © Michel Owusu / CeMM;
The study "Validating the concept of mutational signatures with isogenic cell models" was published in Nature Communications 9 on May 1st, 2018. DOI: 10.1038/s41467-018-04052-8.
Authors: Xueqing Zou*, Michel Owusu*, Rebecca Harris, Stephen P. Jackson, Joanna I. Loizou#, Serena Nik-Zainal# (*These authors contributed equally to this work # Corresponding authors)
The study was funded by the Austrian Academy of Sciences, the European Commission (Marie-Curie Career Integration Grant), the Austrian Science Fund FWF and the Wellcome Trust.
Joanna Loizou received her Ph.D. at the University of Manchester and Sussex with Keith Caldecott, and carried out post-doctoral research at the International Agency for Research on Cancer, Lyon, France with Zhao-Qi Wang and Zdenko Herceg and later at the London Research Institute, CRUK, England with Axel Behrens. She joined CeMM in 2011. http://cemm.at/research/groups/joanna-i-loizou-group/
The mission of CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences is to achieve maximum scientific innovation in molecular medicine to improve healthcare. At CeMM, an international and creative team of scientists and medical doctors pursues free-minded basic life science research in a large and vibrant hospital environment of outstanding medical tradition and practice. CeMM's research is based on post-genomic technologies and focuses on societally important diseases, such as immune disorders and infections, cancer and metabolic disorders. CeMM operates in a unique mode of super-cooperation, connecting biology with medicine, experiments with computation, discovery with translation, and science with society and the arts. The goal of CeMM is to pioneer the science that nurtures the precise, personalized, predictive and preventive medicine of the future. CeMM trains a modern blend of biomedical scientists and is located at the campus of the General Hospital and the Medical University of Vienna. http://www.cemm.at
For further information please contact
Mag. Wolfgang Däuble
Media Relations Manager
Phone +43-1/40160-70 057
Fax +43-1/40160-970 000
[email protected]
http://www.cemm.at
CeMM
Research Center for Molecular Medicine
of the Austrian Academy of Sciences
Lazarettgasse 14, AKH BT 25.3
1090 Vienna, Austria
Media Contact
Wolfgang Däuble
[email protected]
0043-140-160-70057
@CeMM_News
http://www.cemm.oeaw.ac.at
Related Journal Article
http://dx.doi.org/10.1038/s41467-018-04052-8