Credit: Cell
There have been few cancer treatments with such a promising future as using the patient's own immune system. Known as chimeric antigen receptor T-cell therapy, or CAR-T, this treatment uses re-engineered killer T-cells to attack cancer cells, but it also causes potentially deadly side effects. Now, research led by Assistant Professor Wilson Wong (BME) is opening doors to making such therapy safer and more effective.
The research – co-authored by graduate student Jang Hwan Cho and Professor Jim Collins of MIT – is published in the journal Cell. Wong sees the current CAR-T system as having three major flaws: target specificity; strength of response; and lack of adaptive capability, which is essentially the issue of relapse. "Our system has the ability to address those three problems," notes Wong.
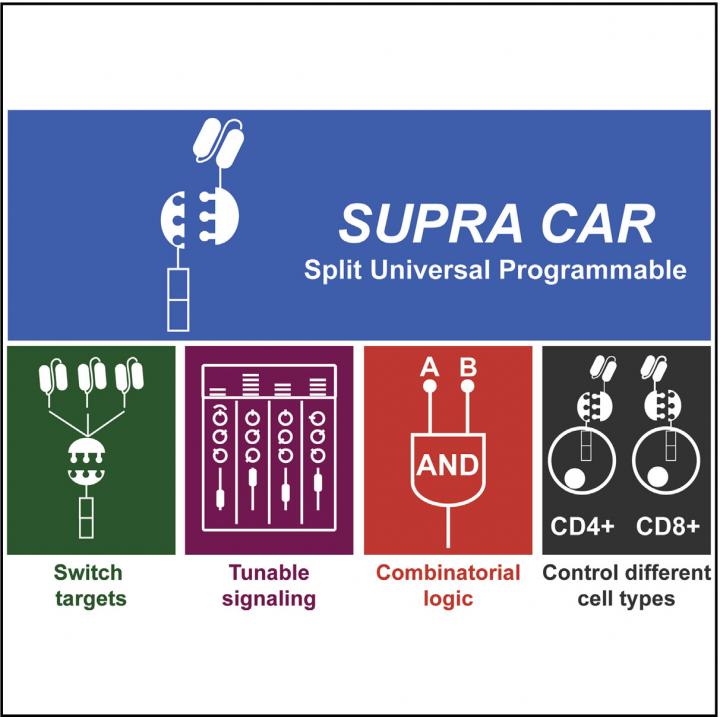
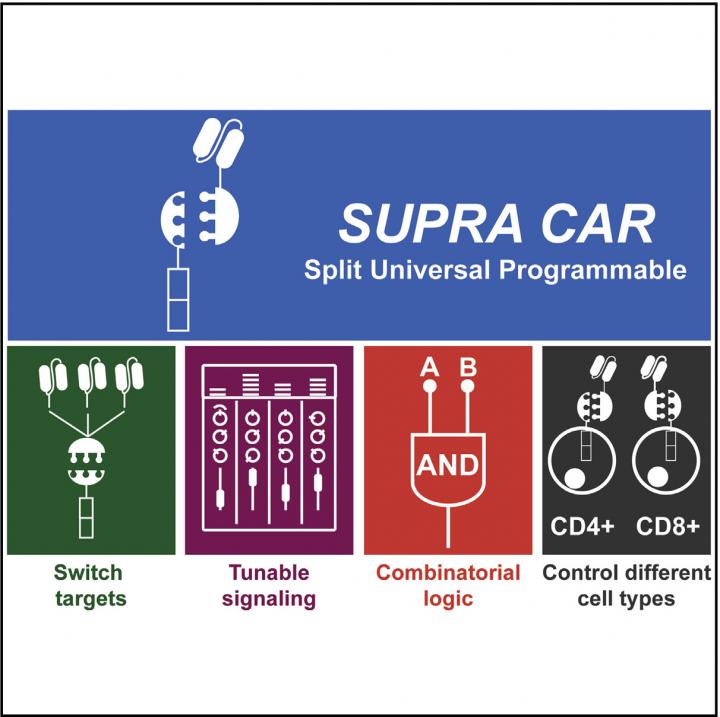
Traditional CAR-T is a treatment engineered for one specific patient to treat one specific type of cancer cell. The new refined system – called split, universal and programmable (SUPRA) CAR-T – can be continuously altered to target different types of cancer cells, turned on and off, and overall offers a significantly more finely tuned treatment than the current therapies.
"Instead of thinking about CAR-T as engineering cells that kill cancer, the way I think about it is as an antibody that drags a killer T-cell with it," says Wong. "What's amazing about it is that once the CAR T-cell binds and activates, it will recruit more T-cells and make copies of itself. Drugs don't do that."
This overwhelming immune response is also what causes the severe side effects. And there have been advances in drug therapy to mitigate these side effects by blocking unnecessary portions of the immune response while still allowing the CAR-T to attack the cancer cells. And the greater number of cancer cells means a stronger immune response. But the SUPRA CAR-T system would let doctors deactivate the entire treatment in case the side effects became too severe.
The original CAR-T can be compared to a charger for a cell phone–one piece that only allows one device to be connected; the SUPRA CAR-T can be compared to two AC adapters that allow for multiple, customizable attachments that can be removed and exchanged at any time. The three features that make SUPRA CAR-T unique are all driven by this ability to split the system into two parts in two ways. And although these three features aren't completely new in the development of new CAR-T systems, the combination of them in one system is.
The normal immune system requires T-cells to sense two targets coming from an invader cell before it attacks it and SUPRA CAR-T works in the same way. Before SUPRA CAR-T attacks cancer cells, it needs to sense that both targets are present on the cell. If you have a lightning cable and a micro USB cable plugged into the adapters, both devices would need to connect to activate the killer T-cell response. If only one is present, the system isn't activated.
SUPRA CAR-T also splits the T-cell from the target-sensing portion of the system–the adapter from the cable. The target on cancer cells is called an antigen and whichever antigen is chosen is sought out by an antibody on the CAR T-cells. The new system breaks apart the T-cell from the antibody and allows for the ability to switch targets; unplugging a lightning cable from an adapter and plugging in a different charging cable.
The ability to switch targets is what can prevent relapse in patients. Cancer cells are smart and will mutate to no longer display the target when they sense the T-cells attacking after attaching to it. The SUPRA CAR-T system allows the T-cells to attack a new target by simply injecting the patient with a new batch of antibodies rather than having to re-engineer the T-cells, which is the most expensive portion of the treatment.
The third feature this split system produces is the ability to finely tune how active the T-cell response, which helps mitigate the dangerous side effects of previous CAR-T systems. By introducing a third component that can block the bonding of the T-cell and the antibody, the SUPRA CAR-T system can be deactivated. The level of deactivation can be tuned by choosing the strength to which this third component binds to the antibody.
Moving forward, Wong hopes CAR-T can become the frontline treatment for cancer that can significantly improve quality of life for patients. He also sees it becoming more. "The way I imagine it is a tricked-out cell," he says. "We want something off-the-shelf so we wouldn't have to make it for every patient, which would bring the cost down, with the capability to switch it on or off. We'd also want it to be able to make certain proteins it might need–proteins that chew up solid-tumor boundaries for example. Lots of sensors, lots of switches, all these bells and whistles that make a very smart cell."
###
Media Contact
Wilson Wong
[email protected]
617-358-6958