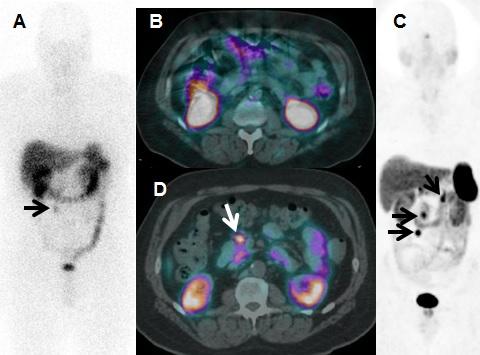
Reston, Va. – A recent study reported in the May issue of the Journal of Nuclear Medicine demonstrates that Ga-68 DOTATATE PET/CT scans are superior to In-111 pentetreotide scans, the current imaging standard in the United States for detecting neuroendocrine tumors (NETS), and could significantly impact treatment management.
NETS occur mostly in the respiratory and digestive tracts and are usually slow-growing. They can be difficult to diagnose, and many treatment options exist. It’s therefore critical to delineate the extent of disease accurately for proper management. While the incidence of NETS is relatively low, with 2.5-5 cases per 100,000 in the United States, data from the National Cancer Institute show a five-fold increase worldwide from 1973 to 2004. NETS can be malignant and, although they comprise less than two percent of gastrointestinal cancers, they are more prevalent than stomach and pancreatic cancers combined.
Ronald C. Walker, MD, corresponding author for the study and professor of clinical radiology and radiological sciences at Vanderbilt University School of Medicine, explains, “Our purpose was to evaluate the safety and efficacy of Ga-68 DOTATATE PET/CT compared to In-111 pentetreotide imaging for diagnosis, staging and re-staging of pulmonary and gastroenteropancreatic neuroendocrine tumors.” With concerns for patient safety, detailed toxicity data were also collected.
The two imaging methods were performed in 78 of 97 consecutively enrolled patients with known or suspected pulmonary or gastroenteropancreatic (GEP) NETs. The study found that Ga-68 DOTATATE PET/CT combined with CT and/or liver MRI changed care in 28 of 78 (36 percent) patients. In addition, Ga-68 DOTATATE PET/CT correctly identified three patients for peptide receptor radiotherapy who had been incorrectly classified by In-111 pentetreotide. Demonstrating no significant toxicity, lower radiation exposure and improved accuracy, the study makes a strong case for the use of Ga-68 DOTATATE imaging over the current standard, where available.
While Ga-68 DOTATATE PET/CT is in widespread use outside of the United States, the U.S. Food and Drug Administration (FDA) has not yet approved its use for the diagnosis, staging and treatment management of NETS. As a result, insurance companies do not cover its use.
Walker says, “Hopefully, our investigation will provide sufficient evidence on the safety and efficacy of Ga-68 DOTATATE to the U.S. FDA to allow approval. If so, then patients throughout the United States could soon have access to a higher-quality scan, allowing better patient management decisions while also lowering radiation exposure and shortening examination time.” Such approval would also open up the possibility of reimbursement for the scans by third-party payers.
These findings could have an impact on the future of nuclear medicine, as well, as Walker explains: “If our evidence results in FDA approval of Ga-68 DOTATATE imaging for routine use in the treatment management of patients with neuroendocrine tumors, it would represent the first approval of a Ga-68-labeled PET imaging radiopharmaceutical in the U.S. This could help pave the way for similar studies to allow approval in the U.S. and elsewhere of other Ga-68-labeled radiopharmaceuticals, such as Ga-68 DOTATOC and Ga-68 PSMA.”
###
Authors of the article “Safety and Efficacy of Ga-68-DOTATATE PET/CT for Diagnosis, Staging and Treatment Management of Neuroendocrine Tumors” include:
– Stephen A. Deppen, MS, PhD, and Gary T. Smith, MD, of the Veterans Affairs Hospital (VA Tennessee Valley Healthcare System) and Vanderbilt University Medical Center, Nashville, Tenn.;
– Eric Liu, MD, of Rocky Mountain Cancer Centers, Denver, Co.;
– Jeffrey D. Blume, PhD; Jeffrey Clanton, DPh; Chanjuan Shi, MD; Laurie B Jones-Jackson, MD; Martin P. Sandler, MD; and Dominique Delbeke, MD, PhD, of Vanderbilt University Medical Center;
– Jordan Berlin, MD, of Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center
– Vipul Lakhani, MD, of the Oregon Medical Group, Springfield, Ore.;
– Richard P. Baum, MD, PhD, of the THERANOSTICS Center for Molecular Radiotherapy and Molecular Imaging (PET/CT), ENETS Center of Excellence, Zentralklinik, Bad Berka, Germany;
– Michael Graham, MD, PhD, of the University of Iowa, Iowa City, Iowa; and
– Ronald C Walker, MD, of the Veterans Affairs Hospital (VA Tennessee Valley Healthcare System), Vanderbilt University Medical Center, and Vanderbilt-Ingram Cancer Center.
Support for this study was provided by the U.S. Department of Veterans Affairs Merit Review: I01BX007080, Society of Nuclear Medicine and Molecular Imaging Clinical Trials Network, and local institutional and philanthropic gifts.
Please visit the SNMMI Media Center to view the PDF of the study, including images, and more information about molecular imaging and personalized medicine. To schedule an interview with the researchers, please contact Laurie Callahan at (703) 652-6773 or [email protected]. Current and past issues of the Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
About the Society of Nuclear Medicine and Molecular Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and medical organization dedicated to raising public awareness about nuclear medicine and molecular imaging, a vital element of today’s medical practice that adds an additional dimension to diagnosis, changing the way common and devastating diseases are understood and treated and helping provide patients with the best health care possible.
SNMMI’s more than 17,000 members set the standard for molecular imaging and nuclear medicine practice by creating guidelines, sharing information through journals and meetings and leading advocacy on key issues that affect molecular imaging and therapy research and practice. For more information, visit http://www.snmmi.org.
Media Contact
Laurie Callahan
[email protected]
@SNM_MI
http://www.snm.org
The post A better way to diagnose and manage neuroendocrine tumors appeared first on Scienmag.





