Credit: Research Funding of Longevity Sciences
Obu, Japan, September 15, 2017 – A new study from the Multimodal Neuroimaging for AD Diagnosis (MULNIAD) study, which is a prospective study implemented at the National Center for Geriatrics and Gerontology (NCGG), provides that hypometabolism in the medial prefrontal areas is specifically associated with Alzheimer's disease-related nutritional problems, and decrease in fat mass may have a key role. This study is published in the Journal of Alzheimer's Disease.
Nutritional problems, especially weight loss, are commonly seen in individuals with Alzheimer's disease (AD); however, the underlying mechanisms are not well understood.
"This study is a very important study to help understand the underlying mechanism of weight loss in patients with mild cognitive impairment and Alzheimer's disease.", says Dr. Takashi Sakurai, corresponding author, head of the Memory Clinic at the NCGG.
This current study is the first to clarify the associations of nutritional status with AD-related brain changes comprehensively by using multi imaging modalities including amyloid-β (Aβ)- positron emission tomography (PET), 18F-fluorodeoxyglucose (FDG)-PET, and structural magnetic resonance imaging.
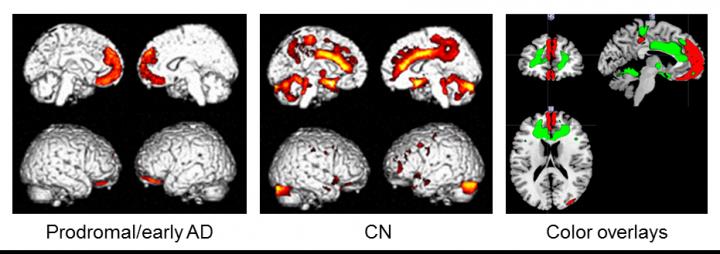
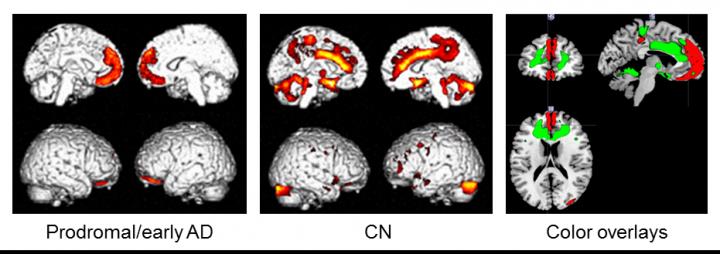
Subjects were 34 Aβ-positive individuals with mild cognitive impairment or early AD (prodromal/early AD), and 55 Aβ-negative cognitively normal (CN) subjects. The associations between nutritional status (body mass index, waist to height ratio, fat mass index, and fat-free mass index) and brain changes were examined by multiple regression analysis using statistical parametric mapping.
In the prodromal/early AD group, nutritional status was significantly positively correlated with regional cerebral glucose metabolism (rCGM) in the medial prefrontal cortices, while different topographical associations were seen in the CN group. Aβ deposition and gray matter volume were not associated with nutritional status. Sub-analysis in the prodromal/early AD group demonstrated that fat mass index, but not fat-free mass index, was positively correlated with rCGM in the medial prefrontal areas.
"These results suggest that hypometabolism in the medial prefrontal areas is specifically associated with AD-related weight loss, and decrease in fat mass may have a key role. However, this cross-sectional study provides preliminary results, so we need further longitudinal investigation considering the fat tissue metabolism including adipokines to deepen our understanding of AD related weight loss", says Dr. Takashi Sakurai. After adjusting for cognitive function, the significant link between fat-mass index and rCGM was preserved.
The study findings of decreased glucose metabolism in medial prefrontal areas in Aβ-positive individuals may explain the underlying mechanism of weight loss in AD.
###
Media Contact
Takashi Sakurai
[email protected]
81-562-462-311
@IOSPress_STM
http://www.iospress.com
Related Journal Article
http://dx.doi.org/10.3233/JAD-170257