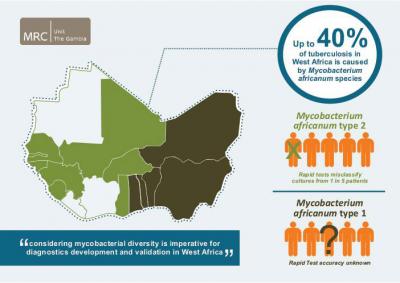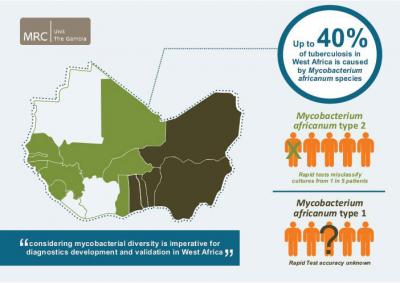
World-wide, Mycobacterium tuberculosis (Mtb) is responsible for the vast majority of tuberculosis (TB) cases. However, there are several other closely related mycobacterial species that cause TB, all part of the Mycobacterium tuberculosis complex (Mtbc). One of them, Mycobacterium africanum (Maf), causes up to 40% of TB cases in West Africa. TB diagnosis across Africa relies largely on tests optimized to detect Mtb. A study led by the Medical Research Council Unit The Gambia and the Antwerp Institute of Tropical Medicine, Belgium, published in PLOS Neglected Tropical Diseases now suggests that in West Africa tests to identify Mtbc in culture miss a substantial fraction of cases, with dire consequences for the patients and for TB control efforts.
Concerned about substantial discrepancies in samples from West Africa between the sputum-based results and the results of rapid tests, Florian Gehre, who is affiliated with MRC and ITM, and colleagues undertook a systematic evaluation of two commonly used rapid TB tests. Both tests detect the product of the mycobacterial mpt64 gene.
The researchers started by comparing the abundance of mpt64 gene product in sputum samples of patients with untreated pulmonary TB caused by Maf 2 (the Maf strain common in The Gambia) or Mtb. Samples from five patients with Maf 2 TB, they found, had about 2.5 times less mpt64 gene product than those from six patients whose disease was caused by Mtb.
They then prospectively analyzed culture isolates from 173 patients with one of the rapid tests, the BD MGITTM TBc ID kit. All of the patients had positive sputum microscopy, and cultures were negative for a second test that detects contamination by unrelated bacteria. Based on molecular analysis, the researchers knew that 122 of the samples were from patients with Mtb TB; the remaining 51 samples from patients with Maf 2 TB.
150 of the samples tested positive on Day zero (the day when mycobacterial growth in culture was first recorded), with 23 (13.2%) testing negative at this time point. The accuracy was much higher (over 90%) for the Mtb samples, compared with less than 80% for the Maf 2 samples. The researchers then ran the tests for another 90 days to see whether and when initially negative samples would turn positive (test instructions recommend reading results between Days zero and ten). At Day 10, 84% of Maf 2 samples tested positive compared with 98% of Mtb samples. By Day 90, 98% of both Mtb and Maf 2 samples tested positive.
Based on these results, 22% of Maf 2 patients, and 10% of Mtb patients would have been wrongly classified as having non-TB mycobacteria if the tests had not been repeated after Day zero. At the end of the 10-day window recommended by the BD MGITTM TBc ID manufacturer, 16% of all Maf 2 samples remained negative, compared with only 2% of Mtb samples.
The researchers repeated the same analysis with all samples that had tested negative on Day zero and a random set of those that had tested positive using the SD Bioline Ag MPT64 RapidTM test. They observed no significant difference between the two tests.
Their findings, the researchers say, "indicate that MPT64 tests need to be cautiously used in settings where Maf 2 is common". However, they also recognize that "given the relatively low cost, limited technical expertise and shorter turnaround time associated with using rapid speciation tests compared to alternative speciation methods, MPT64 rapid tests will likely remain one of the preferred options for timely diagnosis of suspected TB despite the possibility of false negative results", and suggest that "a negative MPT64 result would require confirmation by an alternative method".
Overall, they say, their results emphasize "the need to consider strain diversity during TB product development", and demonstrate that "careful evaluation and validation of novel tests before implementation, especially in regions with geographically restricted Mtbc lineages, such as M. africanum in West Africa, is imperative".
###
Media Contact
PLOS NTDs
[email protected]