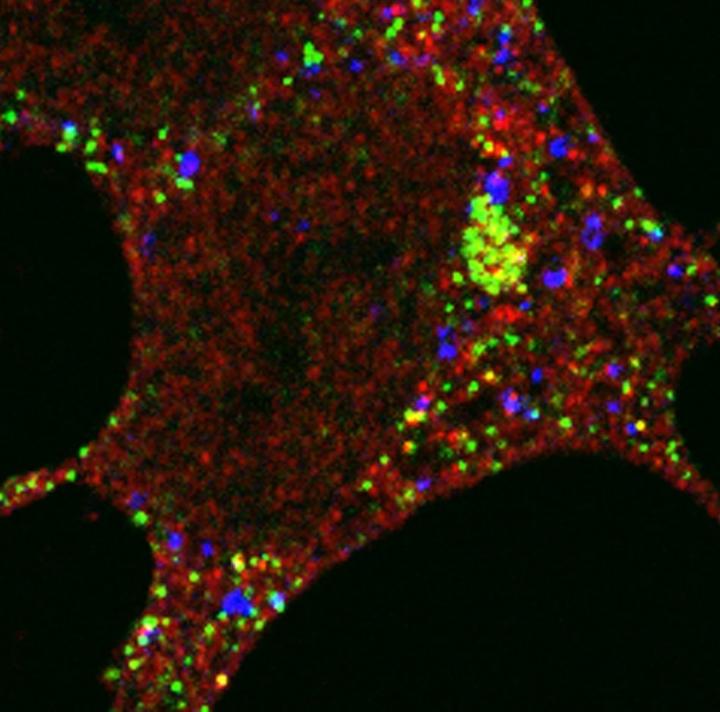
Credit: Sharon Tooze
Scientists have made an important step in understanding how cells keep themselves clean and healthy – a finding that may have implications for combating neurodegenerative diseases and cancer.
One way that our bodies clean out toxic debris and damaged cell components is by a process called autophagy, which means 'self-eating'. Our cells create internal 'recycling bins' called autophagosomes that collect diseased, dead, or worn-out cell parts, strips them for useful bits, and uses the resulting molecules for energy to make new healthy cell parts. When this disposal system stops working properly, it can lead to cancer and diseases like Alzheimer's and Parkinson's.
Researchers at the Francis Crick Institute have uncovered a pathway that controls autophagy, which could potentially be targeted in future to prevent diseases. The research is published in Current Biology.
The team had previously shown that in starved cells that need to recycle nutrients for energy, an important protein required for autophagy, GABARAP, moves from the centrosome – part of the cell that contains structural scaffolds that maintain its shape and enable cell division and movement – to the autophagosome.
In this study, they used visual markers and biochemical tools to see how the autophagy protein gets to where it needs to be. They found that a protein called PCM1 forms a compartment or 'centriolar satellite' which shuttles the autophagy protein from the centrosome to the autophagosome along a scaffold, a bit like a train carriage transporting a person along a railway track. When they deleted the PCM1 gene, the GABARAP autophagy protein's journey to the autophagosome became disorganised. Some GABARAP was degraded by an alternative recycling bin in the cell – the proteasome – and some GABARAP went to different autophagosomes from normal, highlighting the importance of PCM1 in controlling the assembly of the autophagy cell machinery.
"The identification of this new type of autophagosome formed by the disorganised GABARAP tells us that there are unique types of autophagosomes in the cell but we don't yet understand how they would work to prevent disease," says Sharon Tooze, Group Leader at the Francis Crick Institute. "One of the aims of our ongoing research is to manipulate this pathway, to boost cells' ability to keep themselves clean and healthy."
Justin Joachim, post-doctoral fellow at the Francis Crick Institute and first named author of the paper adds: "Our work reveals a previously unknown connection between the centrosome, cell division, shuttle proteins and autophagy and establishes a new regulatory pathway to control autophagy,"
The paper 'Centriolar satellites control GABARAP ubiquitination and GABARAP-mediated autophagy' is published in Current Biology.
###
Media Contact
Greta Keenan
[email protected]
020-379-65252
@thecrick
http://www.crick.ac.uk