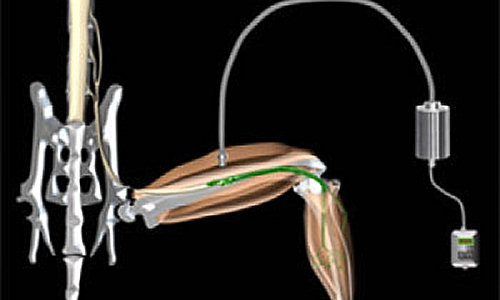
A new way to artificially control muscles using light, with the potential to restore function to muscles paralysed by conditions such as motor neuron disease and spinal cord injury, has been developed by scientists at UCL and King’s College London.
The technique involves transplanting specially-designed motor neurons created from stem cells into injured nerve branches. Photo Credit: Barney Bryson
The technique involves transplanting specially-designed motor neurons created from stem cells into injured nerve branches. These motor neurons are designed to react to pulses of blue light, allowing scientists to fine-tune muscle control by adjusting the intensity, duration and frequency of the light pulses.
In the study, published this week in Science, the team demonstrated the method in mice in which the nerves that supply muscles in the hind legs were injured. They showed that the transplanted stem cell-derived motor neurons grew along the injured nerves to connect successfully with the paralyzed muscles, which could then be controlled by pulses of blue light.
“Following the new procedure, we saw previously paralysed leg muscles start to function,” says Professor Linda Greensmith of the MRC Centre for Neuromuscular Diseases at UCL’s Institute of Neurology, who co-led the study. “This strategy has significant advantages over existing techniques that use electricity to stimulate nerves, which can be painful and often results in rapid muscle fatigue. Moreover, if the existing motor neurons are lost due to injury or disease, electrical stimulation of nerves is rendered useless as these too are lost.”
Muscles are normally controlled by motor neurons, specialized nerve cells within the brain and spinal cord. These neurons relay signals from the brain to muscles to bring about motor functions such as walking, standing and even breathing. However, motor neurons can become damaged in motor neuron disease or following spinal cord injuries, causing permanent loss of muscle function resulting in paralysis
“This new technique represents a means to restore the function of specific muscles following paralysing neurological injuries or disease,” explains Professor Greensmith. “Within the next five years or so, we hope to undertake the steps that are necessary to take this ground-breaking approach into human trials, potentially to develop treatments for patients with motor neuron disease, many of whom eventually lose the ability to breathe, as their diaphragm muscles gradually become paralysed. We eventually hope to use our method to create a sort of optical pacemaker for the diaphragm to keep these patients breathing.”
The light-responsive motor neurons that made the technique possible were created from stem cells by Dr Ivo Lieberam of the MRC Centre for Developmental Neurobiology, King’s College London.
“We custom-tailored embryonic stem cells so that motor neurons derived from them can function as part of the muscle pacemaker device.” says Dr Lieberam, who co-led the study. “First, we equipped the cells with a molecular light sensor. This enables us to control motor neurons with blue light flashes. We then built a survival gene into them, which helps the stem-cell motor neurons to stay alive when they are transplanted inside the injured nerve and allows them to grow to connect to muscle.”
Story Source:
The above story is based on materials provided by University College London, Harry Dayantis.