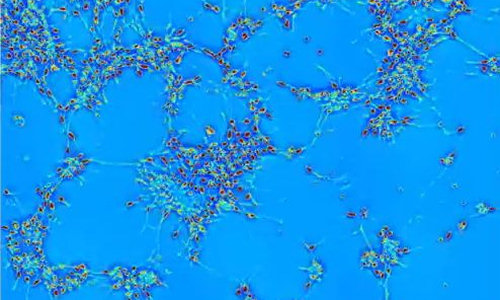
A new technique, developed by researchers in the Quantitative Light Imaging Laboratory at the Beckman Institute at the University of Illinois, provides a method to noninvasively measure human neural networks in order to characterize how they form.
Using spatial light interference microscopy (SLIM) techniques developed by Gabriel Popescu, director of the lab, the researchers were able to show for the first time how human embryonic stem cell-derived neurons within a network grow, organize spatially, and dynamically transport materials to one another.
“Because our method is label-free, we’ve imaged these type of neurons differentiating and maturing from neuron progenitor cells over 12 days without damage,” said Popescu. “I think this (technique) is pretty much the only way you can monitor for such a long time.”
Scientific Reports recently published their paper on the topic, “Label-Free Characterization of Emerging Human Neuronal Networks.”
Using time-lapse measurement, the researchers are able to watch the changes over time. “We’ve been looking at the neurons every 10 minutes for 24 hours to see how the spatial organization and mass transport dynamics change,” said Taewoo Kim, one of the lead authors on the paper.
The SLIM technique measures the optical path length shift distribution, or the effective length of the path that light follows through the sample. “The light going through the neuron itself will be in a sense slower than the light going through the media around the neuron,” explains Kim. Accounting for that difference allows the researchers to see cell activity—how the cells are moving, forming neural clusters, and then connecting with other cells within the cluster or with other clusters of cells.
“Individual neurons act like they are getting on Facebook,” explains Popescu. “In our movies you can see how they extend these arms, these processes, and begin forming new connections, establishing a network.” Like many users of Facebook, once some connections have been made, the neurons divert attention from looking for more connections and begin to communicate with one another—exchanging materials and information. According to the researchers, the communication process begins after about 10 hours; for the first 10 hours the studies show that the main neuronal activity is dedicated to creating mass in the form of neural extensions or neurites, which allows them to extend their reach.
“Since SLIM allows us to simultaneously measure several fundamental properties of these neural networks as they form, we were able to for the first time understand and characterize the link between changes that occur across a broad range of different spatial and temporal scales. This is impossible to do with any other existing technology,” explains Mustafa Mir, a lead author on the study.
The researchers used untreated cells and cells that were treated with lithium chloride, which delays neurite growth. This allowed them to compare the mass of both the treated and untreated cells, and showed that the main growth of cells is during the connection phase, where dendrites are being extended within and between clusters.
“When we first compared the data from the treated and untreated samples, the potential of this technique to answer important questions in neuroscience and developmental biology became clear. Many diseases result in subtle changes in how neural networks organize and behave, and now we have a tool to study these changes in a practical manner,” said Mir.
The work is done in conjunction with a science and technology center sponsored by the National Science Foundation, Emergent Behaviors of Integrated Cellular Systems (EBICS), which is a multi-institutional effort led by MIT, Georgia Tech, and the University of Illinois. The Illinois site is led by Rashid Bashir, from the Beckman 3D Micro- and Nanosystems Group and head of the Illinois Department of Bioengineering.
EBICS is examining the way that complex systems and patterns rise out of relatively simple interactions with a goal of building living, multi-cellular machines that solve real-world problems in health, security, and the environment.
“Through this center in the past four years, we’ve developed a number of tools trying to understand emergence, trying to define what emergence means,” said Popescu. “This paper is a clear example of our progress toward defining and quantifying this important and universal phenomena.
“We developed these methods based on SLIM to understand at what scale the cells get organized, they become predictable. We quantify emergence versus spatial and temporal scales at which order occurs. The formation of a neuron network is a beautiful example of how emergence occurs. You deploy the cells that have nothing to do with one another—they are completely independent. Then, in less than 24 hours they start to talk to one another—operate more as an ensemble, an organized group, rather than as individuals. Using SLIM we can attest that, although structurally, individual cells do not change much over short time scales, it is their arrangement in space and their collective behavior in time that evolves quickly.”
Popescu hopes that his work will help in building machines that can help with health-related questions, including Alzheimer’s, memory-related conditions, and aging. The first step is to identify the deterministic behavior of the neural cells and discover treatments that enhance this predictable behavior.
“I think we have a set of good tools to both structurally and dynamically tell the difference when cells are functioning in various modes now. The plan is to control the predictable part, such as material transport in neurons or beating in heart cells, and hopefully get the cells to accomplish small tasks,” said Popescu.
“Although in this study we have used SLIM to examine neural networks, the technology showcased here can be applied to a wide variety of biological systems, this is only the tip of the iceberg,” said Mir.
“SLIM technology holds tremendous promise for imaging not only cell-based networks, but also whole slices of brain tissue, where the connections laid down during development are preserved and natural functionalities are expressed,” said Martha Gillette, a neuroscientist in the Neurotech Group at the Beckman Institute and collaborator on the study. “Heterogeneities among individual cells of functionally specialized brain regions are emerging as key contributors to healthy versus diseased states. The ability to analyze individual cells within a multicellular micro-environment that preserves the native structural and functional complexity of the brain is a major challenge that can be addressed using SLIM.”
SLIM is currently being installed in the Microscopy Suite at the Beckman Institute in order to allow other researchers access to this imaging technique.
Story Source:
The above story is based on materials provided by Beckman Institute for Advanced Science and Technology.