In a groundbreaking study recently published in Genes & Diseases, researchers from Jilin University have unveiled a pivotal mechanism controlling the metastatic potential of colorectal cancer (CRC) through the molecular interplay between UBR5, an E3 ubiquitin ligase harboring a HECT domain, and Snail, a key transcriptional orchestrator of epithelial-mesenchymal transition (EMT). This discovery sheds new light on how metastatic progression in CRC can be thwarted, offering promising avenues for targeted therapies.
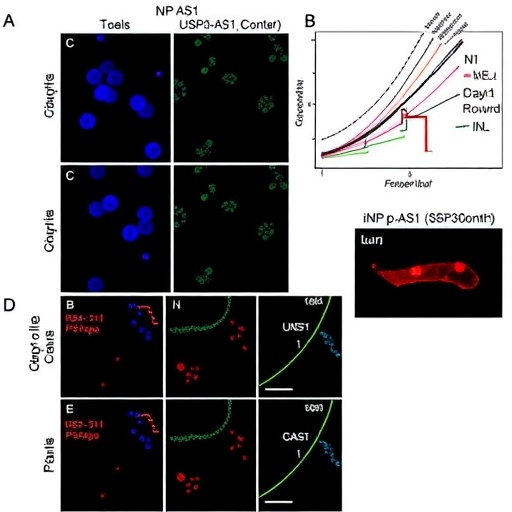
The crux of the research lies in the identification of UBR5 as a critical tumor suppressor that directly modulates the stability of Snail. Utilizing affinity purification coupled with mass spectrometry, the team isolated UBR5 as an exclusive binding partner of Snail, illuminating a previously underappreciated regulatory axis. Notably, this interaction underscores a post-translational checkpoint essential for maintaining cellular phenotype and metastatic restraint.
Extensive bioinformatic analyses employing datasets from TCGA and GTEx revealed a compelling correlation between UBR5 expression and colorectal cancer progression. Particularly striking was the observation in stage II colorectal tumors where UBR5 expression markedly declined whereas Snail levels surged. This inverse relationship suggests that UBR5 depletion potentiates Snail stabilization during crucial early steps of metastasis, aligning with the biological hallmarks of EMT activation.
Delving deeper, the researchers meticulously defined the domain-specific interaction that underpins this regulation. Through a combination of co-immunoprecipitation assays, protein truncation constructs, and molecular docking simulations, they demonstrated that UBR5 engages Snail via its HECT domain (amino acids 2453–2799), which specifically binds to the C-terminal zinc finger domain of Snail (amino acids 151–264). This nuanced specificity is a remarkable divergence from other known E3 ligase interactions within the RING family, positioning UBR5 as a unique modulator with distinct biochemical properties.
Crucially, the UBR5-mediated regulation of Snail involves targeted K48-linked polyubiquitination leading to proteasomal degradation. This ubiquitination process is not arbitrary but contingent on prior phosphorylation of Snail by GSK-3β, thereby integrating multiple post-translational control layers. The integrity of the HECT domain in UBR5, especially residue Cys2768, was demonstrated to be indispensable for catalyzing this ubiquitination reaction, highlighting the precise molecular requirements for functional suppression of Snail.
Loss-of-function experiments unveiled the biological consequences of UBR5 deficiency: stabilization of Snail protein, downregulation of epithelial marker E-cadherin, and enhanced expression of mesenchymal markers. These molecular alterations synergistically confer increased cellular motility and invasiveness, hallmark traits of aggressive metastatic phenotypes. Importantly, these in vitro findings were recapitulated in vivo using xenograft mouse models, where UBR5 deficiency correlated with accelerated tumor growth and invasive behavior into adjacent tissues.
Further validating the critical role of the catalytic site, the researchers engineered a mutant UBR5 variant harboring the C2768S mutation within the HECT domain. This mutant lost the ability to physically interact with Snail and failed to promote its degradation. In vivo, tumors expressing this catalytically impaired UBR5 mutant exhibited pronounced aggressiveness and tissue infiltration compared to those expressing wild-type UBR5, which displayed significant tumor regression and encapsulation, affirming the therapeutic relevance of UBR5’s enzymatic function.
From a clinical perspective, interrogation of large CRC patient cohorts reinforced the tumor suppressive role of UBR5, revealing its consistent downregulation in colorectal cancer tissues relative to normal counterparts. More importantly, elevated UBR5 expression levels correlated positively with relapse-free survival, positioning UBR5 not only as a molecular guardian against metastasis but also as a promising prognostic biomarker.
This study elegantly elucidates the UBR5-Snail axis as a linchpin in the regulation of epithelial plasticity and metastatic progression. The requirement for GSK-3β-mediated phosphorylation prior to UBR5-dependent ubiquitination signifies an intricate multi-step regulatory cascade, emphasizing the interplay of kinase and ligase activities in EMT control. Such insights enrich our understanding of the molecular pathology underlying CRC and open potential strategies for therapeutic intervention that restore or mimic UBR5 function.
The identification of the zinc finger motif of Snail as the interacting domain further expands the paradigm of transcription factor regulation by ubiquitin ligases. This structural specificity accounts for the lack of interaction with Snail’s close homolog Slug (Snail2), reinforcing the selectivity of the regulatory network and its potential for targeted drug design.
Technologically, the integration of biochemical assays, protein engineering, clinical genomics, and in vivo tumor models offers a robust multi-dimensional platform for dissecting cancer metastasis mechanisms. The combinatorial use of molecular docking simulations validated the physical plausibility of the UBR5-Snail binding interface, exemplifying the power of computational tools in complementing experimental biology.
In conclusion, the work from Jilin University delineates a sophisticated post-translational checkpoint wherein UBR5 operates as a crucial suppressor of colorectal cancer metastasis by orchestrating the degradation of Snail. Its catalytic competency, governed by Cys2768 within the HECT domain, emerges as a molecular switch balancing epithelial identity against mesenchymal transition. Continued exploration of this axis holds significant promise for the development of novel anti-metastatic therapeutics and prognostic markers in colorectal and potentially other cancers.
Subject of Research: Colorectal Cancer, Metastasis, Epithelial-Mesenchymal Transition (EMT), Ubiquitination
Article Title: Identification of UBR5 as a Critical E3 Ligase Modulating Snail Stability and Metastatic Progression in Colorectal Cancer
News Publication Date: Not provided
Web References: http://dx.doi.org/10.1016/j.gendis.2025.101679
Image Credits: Xinyue Zhao, Ruiying Liu, Zhihui Han, Zehao Li, Ling Mei, Yuyang Liu, Xueqi Fu, Yue Jin
Keywords: Colorectal cancer, Metastasis, UBR5, Snail, E3 Ubiquitin Ligase, HECT domain, EMT, Post-translational modification, GSK-3β, Zinc finger domain, Protein degradation, Tumor suppressor
Tags: colorectal cancer metastasis regulationE3 ubiquitin ligase function in cancerepithelial-mesenchymal transition in CRCmolecular pathways of CRC progressionpost-translational modification in metastasisSnail protein stabilization in cancerSnail transcription factor in EMTtargeted therapies for colorectal cancerTCGA colorectal cancer data analysistumor suppressor genes in metastasisUBR5 role in colorectal cancerUBR5-Snail interaction mechanism