A groundbreaking review led by scientists at the University of Maryland School of Medicine is shedding new light on the intricate role genetic ancestry plays in the biology and treatment response of head and neck squamous cell carcinoma (HNSCC). This study reveals that genetic ancestry, rather than self-identified race, significantly influences tumor behavior, mutation patterns, and patient outcomes, opening new avenues for precision oncology tailored to diverse genetic backgrounds.
Head and neck squamous cell carcinoma, a group of biologically aggressive tumors found in the oral cavity, pharynx, and larynx, has long been associated with external lifestyle risk factors including tobacco use, alcohol consumption, and human papillomavirus (HPV) infection. However, disparities in survival rates have persisted, with African-American patients showing markedly worse outcomes—living on average only 2.5 years post-diagnosis compared to nearly 5 years for their European American counterparts. The University of Maryland team delved deeper into the molecular underpinnings of these disparities by analyzing comprehensive genomic data.
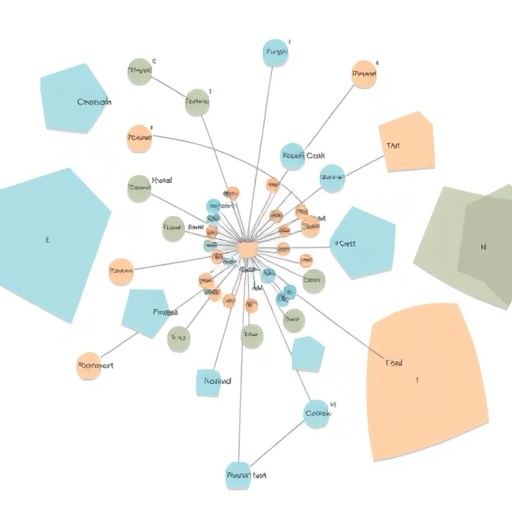
Leveraging The Cancer Genome Atlas (TCGA), the largest repository of molecular profiles from cancer patients worldwide, the investigators systematically examined tumor data from over 500 patients. Crucially, they emphasized genetic ancestry markers—unique segments of DNA inherited from distinct global populations—over self-reported racial identity to uncover biologically relevant distinctions in tumor evolution, mutational landscapes, and gene expression. Their systematic review elucidated that genomic ancestry shapes the DNA alterations driving tumor proliferation, metastasis potential, and therapeutic resistance.
Through detailed bioinformatic analyses, the research team identified a spectrum of genetic alterations enriched in tumors depending on the patient’s ancestral background. Specific DNA copy number variations, gene mutations, and epigenetic modifications demonstrated ancestry-specific patterns. These findings suggest that tumors developed in different populations diverge not merely due to environmental exposures or social determinants but also due to underlying genomic architecture influencing tumor pathophysiology and drug susceptibility.
One of the most striking implications of this work is the reinforcement that complex social factors, such as access to healthcare and lifestyle behaviors, though undeniably impactful, do not wholly account for observed disparities in clinical outcomes. The biological diversity encoded by ancestry must also be incorporated into treatment design. Precision medicine approaches that consider these genomic distinctions hold promise to optimize therapy efficacy, minimize resistance, and ultimately improve survival rates among underrepresented populations.
Madeleine Ndahayo, the study’s lead author and a student researcher at the Institute for Genome Sciences (IGS), and senior author Dr. Daria Gaykalova emphasized the necessity of integrating genomics and social science approaches. Their collaboration illustrates that a multidimensional understanding incorporating both inherited biological variation and social context is essential to eradicate long-standing inequities in head and neck cancer prognosis.
By advancing the fundamental understanding of how ancestry-linked genomic variation influences tumor biology, this review challenge’s the oncology community to rethink clinical trial design, biomarker discovery, and therapeutic targeting strategies. Addressing the molecular heterogeneity shaped by ancestral backgrounds can lead to the development of novel diagnostic tools and personalized treatment regimens that transcend traditional categorizations of race, offering more precise interventions aligned with each patient’s unique tumor profile.
Furthermore, the study highlights the importance of expanding genomic databases to include a wider array of populations, enabling more comprehensive analyses that capture the global diversity of tumor genomes. Efforts to incorporate underrepresented groups will be vital to ensure equitable implementation of genomic medicine and prevent amplification of disparities through biased data sets.
This pioneering work was supported by funding from the American Cancer Society, the National Institute of Dental and Craniofacial Research, and the National Cancer Institute. It reinforces the mission of the University of Maryland’s Institute for Genome Sciences and the Greenebaum Comprehensive Cancer Center to foster innovative, inclusive research that translates into tangible improvements in cancer care.
The implications of this research extend beyond head and neck cancer, underscoring a paradigm shift whereby genetic ancestry is recognized as a fundamental variable in cancer genomics and precision therapy development. Future clinical protocols may routinely incorporate genomic ancestry assessments to guide treatment choices, predict therapy responses, and monitor disease progression with unprecedented accuracy.
In summary, this review published in Cancer and Metastasis Reviews signals a transformative step toward dismantling cancer health disparities by unveiling the crucial role of genomic ancestry in shaping tumor biology. The findings emphasize that integrating genetic ancestry with socioeconomic factors is indispensable for the next generation of precision oncology strategies, ultimately aiming to deliver equitable and effective care for all patients irrespective of background.
Subject of Research: People
Article Title: The Impact of Genomic Ancestry on Tumor Genomics in Head and Neck Squamous Cell Carcinoma
News Publication Date: 30-Jan-2026
Web References: 10.1007/s10555-026-10312-7
References: Cancer and Metastasis Reviews
Keywords: Head and neck cancer, Genetics
Tags: African American cancer patient outcomesdisparities in cancer survival ratesgenetic ancestry and cancer outcomesgenomic data in cancer researchhead and neck squamous cell carcinoma researchinfluences of ancestry on cancer treatmentlifestyle factors in cancer riskmolecular underpinnings of cancer disparitiesprecision oncology and genetic backgroundssignificance of genetic markers in oncologyThe Cancer Genome Atlas analysistumor behavior and mutation patterns