Recent advancements in biomedical research have unveiled complex interactions between biological factors and drug metabolism. A newly published study by Zhang, Wang, Liu et al. offers significant insights into these interactions, particularly addressing how sex differences influence the effects of strychnine intoxication in rats. This groundbreaking research combines metabolic kinetics and metabolomics to elucidate variations that could inform clinical practices and drug development. The findings hold promise for a deeper understanding of how male and female biological mechanisms respond differently to toxic compounds.
Strychnine, a highly toxic alkaloid, serves as the focal point of this study. Known for its stimulant effects on the central nervous system, strychnine poses serious risks and has been used in toxicological research to understand the pathways through which recognized toxins impact health. The researchers meticulously designed their experiments to investigate how the sex of the subjects influenced the metabolic response to strychnine. By employing both metabolic kinetics and metabolomic profiling, they set out to reveal patterns that would otherwise go unnoticed in a less detailed analysis.
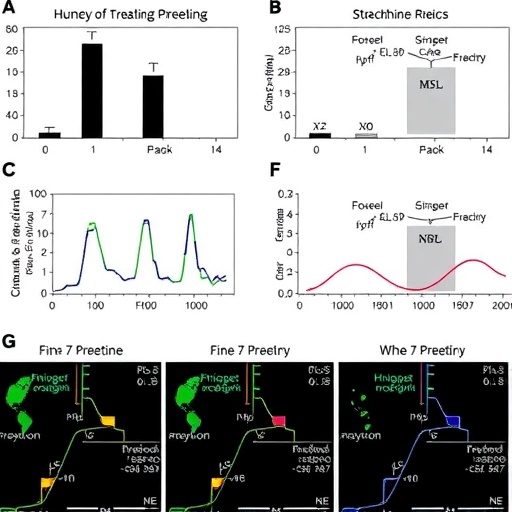
Metabolic kinetics provides insights into the rates at which substances are absorbed, distributed, metabolized, and excreted from the body. This framework helps researchers assess how different biological systems process compounds and can reveal crucial insights into the onset of toxicity and recovery pathways. The study meticulously analyzed these kinetic parameters in both male and female rats, highlighting pronounced differences in how each sex metabolizes strychnine, which, until now, had not been thoroughly addressed in existing literature.
Moreover, metabolomics, the comprehensive study of metabolites in a biological sample, complements the kinetic data by providing an extensive profile of metabolic changes occurring as a result of strychnine exposure. Using advanced analytical techniques such as mass spectrometry and nuclear magnetic resonance spectroscopy, the researchers could identify specific metabolites that exhibited sex-dependent variations in response to the toxin. This dual approach—merging kinetic modeling with metabolomic analysis—allowed for a thorough understanding of the biological pathways involved.
The results demonstrated notable distinctions in how male and female rats metabolized strychnine, providing critical insights into the underlying biological mechanisms of these differences. The team found that males exhibited more rapid clearance of the toxin, attributed to higher activity levels of specific hepatic enzymes responsible for drug metabolism. Conversely, female rats displayed a delayed response, which may relate to different hormonal influences and metabolic rates. Such disparities in metabolism underscore the importance of considering sex as a significant biological variable in pharmacology and toxicology.
These findings are not simply of academic interest. The implications extend into clinical realms, particularly in drug development and treatment strategies for poisoning cases. Knowledge of how sex influences metabolism can guide clinicians in tailoring their approaches when treating patients who have been exposed to toxic substances like strychnine. Thus, this research could pave the way for developing sex-specific antidotes or therapeutic strategies, enhancing the efficacy of interventions in clinical toxicology.
Furthermore, the metabolic profiling revealed several specific metabolites that were significantly altered in concentration following strychnine exposure, with notable differences between sexes. These metabolites may serve as potential biomarkers for susceptibility to strychnine poisoning or for monitoring recovery from intoxication. Identifying such biomarkers could be invaluable in enhancing our understanding of toxicological responses and improving patient outcomes after exposure to such hazardous compounds.
In addition to its immediate relevance to toxicology and drug metabolism, the study opens avenues for further research into the interplay between sex, metabolism, and susceptibility to various toxins. As our understanding of metabolic pathways deepens, researchers may uncover critical connections that inform public health policies and individual treatment protocols related to toxic exposures. Such revelations could also contribute to the broader field of personalized medicine, where treatment plans are tailored based on individual metabolic profiles and biological sex.
The study’s comprehensive nature showcases the necessity of integrating multiple methodological perspectives in biomedical research. By addressing the critical variable of sex, the authors demonstrate that neglecting such biological differences can lead to incomplete or misleading conclusions in the field of pharmacology. Future research will undoubtedly benefit from similar integrative approaches, broadening the horizon of what we know about gender differences in health and disease.
In summary, Zhang and colleagues’ research presents a compelling argument for the importance of considering biological sex in both experimental design and clinical application when studying toxicological effects. The findings not only provide immediate implications for the treatment of strychnine poisoning but also encourage a reassessment of established methodologies across various fields of biological research. This pivotal work serves as a reminder that our understanding of human health and disease is enriched by exploring the nuanced ways in which sex and biology interact with environmental factors.
Overall, this study situates itself at the forefront of contemporary research, shedding light on an area that has been relatively underexplored. The potential impact of these findings resonates across multiple disciplines, from toxicology and pharmacology to broader public health initiatives. As the scientific community continues to delve into the intricate landscape of drug metabolism, the importance of a sex-based lens will become increasingly apparent.
The ongoing exploration into the effects of toxins such as strychnine will likely unveil further complexities and considerations, reinforcing the necessity of nuanced and inclusive research. Zhang and his co-authors have set a commendable example of how integrating advanced techniques with a strong emphasis on biological variables can lead to significant advancements in our understanding of health and disease.
This work marks a significant step toward a more comprehensive understanding of the relationship between biological sex and drug metabolism, an area that bears crucial implications for both scientific research and clinical practice. Researchers and healthcare professionals alike must prioritize these insights as they continue to unravel the complexities of human biology.
As we move forward, it will be imperative for future studies to build upon these findings, fostering a scientific landscape where the nuances of biological differences are not only acknowledged but also utilized to enhance our methodologies and improve health outcomes.
In conclusion, the study by Zhang and colleagues sets a benchmark for future explorations into sex differences in toxicology and metabolism. By championing a multifaceted approach that combines metabolic kinetics with metabolomic analysis, this research serves as a vital contributor to the ever-evolving dialogue surrounding health disparities and personalized medicine in the realm of toxicology.
Subject of Research: Metabolic Kinetics and Metabolomics in Strychnine-Intoxicated Rats
Article Title: Analysis of sex difference in strychnine-intoxicated rat based on the combination of metabolic kinetics and metabolomics.
Article References: Zhang, W., Wang, C., Liu, H. et al. Analysis of sex difference in strychnine-intoxicated rat based on the combination of metabolic kinetics and metabolomics. Biol Sex Differ 16, 100 (2025). https://doi.org/10.1186/s13293-025-00784-7
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s13293-025-00784-7
Keywords: Strychnine, Metabolic Kinetics, Metabolomics, Sex Differences, Toxicology, Pharmacology, Biomarkers, Personalized Medicine.
Tags: biological mechanisms of male and female responsesbiomedical research on intoxication effectscentral nervous system stimulantseffects of toxic compounds on healthgender-specific drug responsesinsights into clinical practicesmetabolic kinetics in toxicologymetabolomics and sex variationssex differences in drug metabolismstrychnine intoxication in ratstoxicological research advancementsunderstanding toxic compound pathways