Recent research has unveiled a complex and dynamic interplay within the cellular environment of colorectal cancer, illustrating how specific molecular pathways can significantly impact the malignant progression of this disease. A key study by Liu and Zhou delves into the positive feedback loop involving IGF2BP3, IL6ST, and STAT3, shedding light on the underlying mechanisms that may offer new insights for therapeutic interventions against colorectal cancer.
Colorectal cancer remains one of the leading causes of cancer-related deaths worldwide, with a grim prognosis for advanced stages of the disease. Despite advancements in screening and treatment, the prognosis remains suboptimal for many patients. The pathway identified in this research highlights the need for an intricate understanding of the molecular events that drive tumorigenesis in colorectal tissue. Such clarity can illuminate potential targets for new pharmacological interventions, providing hope for more effective treatment strategies in the fight against this prevalent cancer.
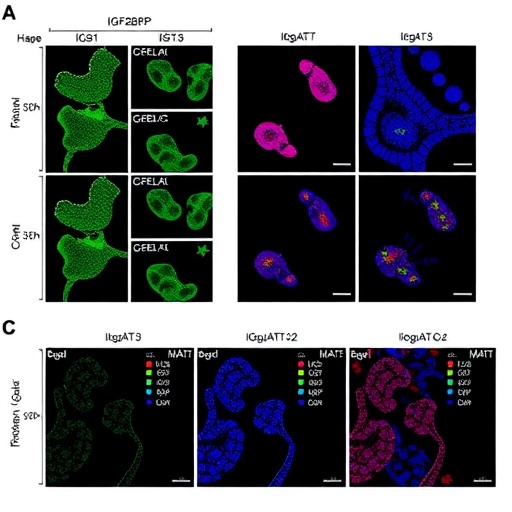
The insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) emerges as a pivotal player in colorectal cancer progression. It has been associated with tumor growth and metastasis, functioning as an oncofetal protein that influences various cellular processes. Through its binding to target mRNAs, it regulates their stability and translation, ultimately promoting cancer cell proliferation and survival. The research expands on existing knowledge by demonstrating that IGF2BP3 acts in concert with IL6ST and STAT3 to create a self-amplifying loop that enhances tumor aggressiveness.
IL6ST, or the interleukin 6 signal transducer, is a critical component of the inflammatory response. Recent findings illustrate its role not just in normal tissue repair and healing, but also in tumor biology. The connection between IL6ST and colorectal cancer progression is underscored by the cytokine milieu within the tumor microenvironment, effectively facilitating a pro-tumorigenic state. The study posits that high levels of IL6ST can lead to increased activation of the STAT3 pathway, further driving the malignant behavior of colorectal cancer cells.
The activation of the STAT3 transcription factor is at the heart of numerous oncogenic processes. When IL6ST binds its ligands, it activates the JAK-STAT signaling cascade, culminating in the phosphorylation of STAT3. This activation allows STAT3 to translocate to the nucleus, driving the expression of genes critical for cell proliferation, survival, and invasion. The elucidation of this pathway by Liu and Zhou positions STAT3 as not only a biomarker of disease progression but a potential therapeutic target that could interrupt the feedback loop fueling cancer advancement.
The study’s findings hold substantial implications for future research and clinical practice. By characterizing the molecular events within this feedback loop, the research delineates a potential roadmap for novel therapeutic strategies aimed at disrupting IGF2BP3, IL6ST, and STAT3 interactions. Targeted therapies that could downregulate or inhibit these molecules may render cancer cells more susceptible to conventional treatments, potentially improving patient outcomes.
Innovations in precision medicine also stand to benefit from this research. Understanding the genetic and molecular underpinnings of colorectal cancer could pave the way for personalized therapeutic approaches. By tailoring treatment based on the specific molecular alterations present in a patient’s tumor, oncologists may be able to enhance treatment efficacy while minimizing adverse effects.
Furthermore, the exploration of the tumor microenvironment as influenced by factors like IGF2BP3 and IL6ST provides a broader perspective on colorectal cancer pathology. By recognizing the importance of extracellular signals and their contribution to tumor development, researchers can investigate combinatorial therapies aimed at reprogramming the tumor stroma to provoke an anti-tumor immune response. This holistic outlook is essential as it acknowledges the multi-faceted nature of cancer, emphasizing that effective treatment must address both tumor cell behavior and the surrounding cellular context.
As studies on colorectal cancer continue to evolve, the integration of findings such as those by Liu and Zhou will be critical. The positive feedback loop they describe represents a crucial nexus of pathways that intertwine to promote tumorigenesis—a target rich in potential for therapeutic exploitation. The science of cancer treatment is on the cusp of a paradigm shift, where molecular insights translate to clinical reality, offering renewed hope to colorectal cancer patients.
The compelling data presented in this groundbreaking study not only expands the scientific community’s understanding of colorectal cancer progression but also lays down a challenge to cancer researchers and clinicians alike. Identifying and targeting these feedback mechanisms could represent a significant leap forward in the fight against colorectal cancer, emphasizing the need for relentless exploration in the field of cancer biology.
The urgent call for innovative solutions to combat colorectal cancer is further emphasized by the study’s implications on public health strategies. With rising incidence rates of colorectal cancer among younger populations, there is an imperative to translate these molecular findings into actionable prevention and treatment strategies. The realization that specific molecular pathways can dictate cancer fate necessitates a re-evaluation of screening practices and treatment modalities to incorporate latest research advancements.
As the fight against colorectal cancer continues, studies like the one by Liu and Zhou underscore the importance of multidisciplinary collaboration in tackling complex biological problems. The integration of molecular biology, clinical insights, and therapeutic innovations will drive progress in developing more effective interventions. Moving forward, the scientific community must synergize its efforts to ensure that such pivotal discoveries translate from the bench to bedside, ultimately benefiting patients battling this formidable disease.
The positive feedback loop highlighted in this research exemplifies the intricate network of interactions that govern cancer biology. With the potential implementation of targeted therapies that disrupt such loops, a new frontier in colorectal cancer treatment could emerge. As we venture into this new territory, sustained investigation and commitment to understanding these processes will be crucial in advancing personalized medicine and improving patient outcomes for those affected by colorectal cancer.
Subject of Research: The role of the IGF2BP3/IL6ST/STAT3 feedback loop in facilitating malignant progression in colorectal cancer.
Article Title: Positive feedback loop of IGF2BP3/IL6ST/STAT3 facilitates malignant progression in colorectal cancer.
Article References:
Liu, P., Zhou, X. Positive feedback loop of IGF2BP3/IL6ST/STAT3 facilitates malignant progression in colorectal cancer.
J Transl Med (2025). https://doi.org/10.1186/s12967-025-07447-6
Image Credits: AI Generated
DOI: 10.1186/s12967-025-07447-6
Keywords: Colorectal cancer, IGF2BP3, IL6ST, STAT3, malignant progression, feedback loop, molecular pathways, targeted therapy, personalized medicine.
Tags: advanced colorectal cancer prognosiscancer-related deaths worldwidecolorectal cancer progression mechanismsIGF2BP3 in colorectal cancerIL6ST STAT3 signaling pathwaymetastasis in colorectal cancermolecular pathways in cancermRNA-binding proteins in tumor growthoncofetal proteins in cancerpharmacological targets for cancer treatmenttherapeutic interventions for colorectal cancertumorigenesis in colorectal tissue