When President Barack Obama announced the $100 million neuroscience initiative called Brain Research through Advancing Innovative Neurotechnologies (BRAIN) in April, the journal Nature called it “a bold bid for the neuroscientist’s ultimate challenge,” but pointed out that “if researchers are to make sense of the frenzy of electrical signals coursing through the brain’s circuits, they will need to record simultaneously from as many neurons as possible,” a capability that has been sharply limited by available technology.
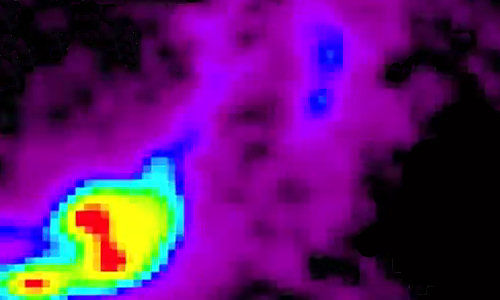
But School of Medicine scientists have now made significant strides toward meeting this challenge by inserting a fluorescent protein in neurons that emits light of varying intensity to mirror changes in electrical activity within the cells. As reported online August 8 in the journal Cell, using optical detectors, the scientists could precisely measure electrical signals in complex neural circuits in a living fruit fly.
“Electrical signals are the language the nervous system uses to transmit information,” says Vincent A. Pieribone, Ph.D., professor of cellular and molecular physiology and of neurobiology and an author of the new paper. “Now we can look at this electrical information optically and non-invasively.”
Achieving the ultimate goals of brain, “to better understand how [humans] think, learn, and remember” and to apply these insights to neurological and psychiatric disease, will first require a deep understanding of simpler nervous systems—those of worms, flies, zebrafish, or mice, for instance—and alternatives to the electrode, the neurophysiologists’s staple tool of the past half-century. Recording electrodes “always cause damage, and are rejected by the brain,” Pieribone says, and in living systems there are physical limits that constrain the number and proper placement of electrodes.
Other researchers have recently reported successes in the optical measurement of neural activity in zebrafish. But co-author Michael N. Nitabach, Ph.D., J.D., associate professor of cellular and molecular physiology and of genetics, says that the method used by these scientists, which tracks calcium levels in neurons, provides only an indirect measure of electrical activity, while the tools developed at Yale provide a precise, direct measure.
Pieribone’s specialty is collecting fluorescent proteins where they occur naturally, such as in deep sea tropical fish. When expressed in neurons as so-called genetically encoded fluorescence voltage indicator proteins (GEVIs), these proteins can serve as visual indicators of electrical activity. Pieribone and other neuroscientists have been engineering gevis for the past 15 years, but they have all turned out to be duds when moved from cell culture to the brains of living animals. “None of them have had sufficient signal size” to be useful, says Nitabach, also a faculty affiliate of the Program in Cellular Neuroscience, Neurodegeneration and Repair.
The GEVI described in the Cell paper, dubbed ArcLight, has a fortuitous mutation that “showed up by accident, like divine intervention,” says Pieribone. This genetic alteration gives ArcLight a strong and exquisitely sensitive fluorescence signal that directly reflects the membrane voltage of the neuron in which it is expressed: it gets dimmer as the voltage rises, and brighter when the voltage diminishes.
Genetically engineering a brain that glows in response to voltage changes may seem innovative enough on its own, but Pieribone knew that ArcLight would only be useful if it reflects brain activity as well as recording electrodes, the gold standard in neuroscience research. To this end, Pieribone collaborated with Nitabach, an expert on the nervous system of the fruit fly Drosophila melanogaster.
Having screened hundreds of fluorescing constructs over the years, Pieribone was stunned when he and Nitabach performed their initial experiments. “The first time we recorded ArcLight in Drosophila I thought there must be something wrong with the traces, because they looked too good,” says Pieribone. “You seldom get those kinds of surprises where things work better than imagined.”
Nitabach and Pieribone decided to express ArcLight in a group of neurons that are well-characterized and known to regulate the fly’s circadian clock. In simultaneous electrode recordings and optical imaging of fluorescing neurons, they showed that results from ArcLight are consistent with those using wires to poke nerve cells. As a bonus, they showed for the first time something that had been suspected in the field but not proven, that the membranes of these cells are more active in the morning than in the evening. “This can’t be recorded any other way,” says Nitabach. “It’s like opening uncharted territory.”
The ArcLight signal is faster than calcium detectors, and there are subtle electrical events that are significant for neural processing that calcium-based methods, and even electrodes, miss entirely, says Nitabach. Action potentials, the Morse code of neurons seen as spiking voltage changes on recordings, are important, he says, but non-spiking signal propagation along neural branches and the synaptic input to neurons that doesn’t reach the threshold to generate an action potential each comprise an little-understood but fundamental part of the neural calculus. These small voltage fluctuations are undetected when the signal of interest is the binary “spike-or-silence” from the neuron’s cell body, the only part of a neuron accessible with an electrode.
ArcLight allows imaging of discrete parts of neurons, a key to understanding this hidden neural computation. “A lot of the information processing and electrical integration is happening in distal parts of the cell that you can’t put an electrode on,” says Nitabach. “With ArcLight you can measure membrane voltage directly at those otherwise inaccessible locations.” The neural volume that can be studied with optical imaging compared with electrodes is also expanded, because light can penetrate deeper into brain tissue without damaging it.
“Seeing electrical activity in the brain directly is a long-standing dream that now seems tangible,” says Gero A. Miesenböck, M.D., director of the Center for Neuronal Circuits and Behavior at the University of Oxford and a former associate professor of cell biology at the School of Medicine. While at Yale, Miesenböck pioneered optogenetics, the use of light to control behavior via genetically encoded photosensitive components in neurons. What Pieribone, Nitabach, and colleagues have done is the flip side of optogenetics, using light, or fluorescence, to visualize neural activity, which Miesenböck calls “an important milestone.” While ArcLight is the most favorable GEVI available at the moment, says Miesenböck, a big challenge still remains in improving optical instrumentation for even better localization of neural signals in both space and time.
Pieribone envisions the two strands of optogenetics coming together in a way that would allow simultaneous optical control and recording of the nervous system. This dovetails well with the ultimate goal of the brain initiative, creating a full circuit diagram of the brain. “Right now we have lots of snapshots, but we don’t know how the nervous system processes things from start to finish,” Pieribone says. An understanding of the whole loop, from sensing to action, in flies or worms is required before the same questions can even be broached for human brains.
Progress on this front should now accelerate considerably, as ArcLight flies have been distributed to numerous labs and the genetic construct is freely available online. Both Pieribone and Lawrence B. Cohen, Ph.D., professor of cellular and molecular physiology, are poised to release results using ArcLight in the mouse brain. “The mutation of ArcLight turned out to be a big hit,” says Pieribone. “I feel this represents a revolution in the way we’re studying the brain.”
Story Source:
The above story is based on materials provided by Kavli Institute for Neuroscience, Amanda Alvarezi.