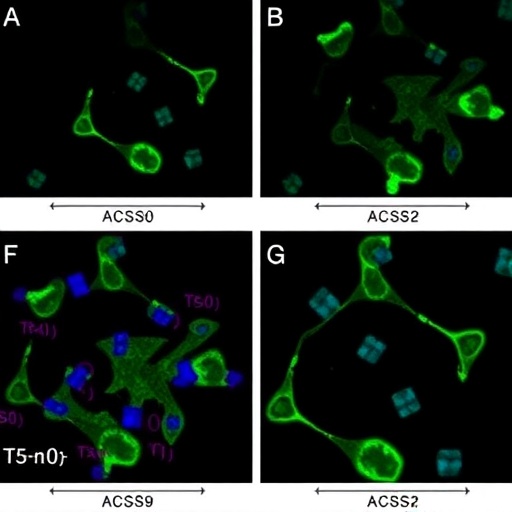
A recent study investigating the role of ACSS2 in ovarian cancer cells has revealed startling insights into the mechanisms underpinning cancer proliferation and invasiveness. The research, conducted by Mroweh et al., focused specifically on the hypoxic conditions that characterize many solid tumors. Under such conditions, cancer cells often adapt and evolve, becoming increasingly aggressive and resistant to conventional therapies. This study highlights the ASCC2 protein’s crucial role in fostering the growth and invasive properties of SKOV-3 and PA-1 ovarian cancer cell lines, illuminating a potential target for therapeutic intervention.
The significance of hypoxia in the tumor microenvironment cannot be overstated. Low oxygen levels can lead to changes in cellular metabolism that favor rapid proliferation. Cancer cells frequently hijack signaling pathways related to hypoxia, enabling them to survive and thrive even when oxygen is scarce. The findings of Mroweh et al. underscore this adaptation, suggesting that ACSS2 serves as a pivotal mediator in the metabolic reprogramming relevant to ovarian cancer progression.
The study meticulously examined the expression of ACSS2 in various ovarian cancer cell lines, identifying a marked increase in expression under hypoxic conditions. Not only does this protein appear to support cell survival, but it also enhances the invasive characteristics commonly associated with malignancy. The researchers employed a suite of techniques, including Western blot analysis and quantitative PCR, to confirm their hypothesis about ACSS2’s role in ovarian cancer.
Moreover, the correlation between ACSS2 expression and hypoxic conditions suggests that this protein could be acting as a survival factor for ovarian cancer cells, effectively helping them cope with metabolic stress. This revelation opens new avenues for targeted therapies aiming to inhibit ACSS2, potentially stunting cancer growth and hindering metastasis.
Furthermore, the study delves into the metabolic pathways involved, specifically focusing on how ACSS2 influences fatty acid metabolism. The protein appears to facilitate the conversion of acetate to acetyl-CoA, a crucial substrate in the biosynthesis of lipids and maintenance of energy homeostasis. In the context of hypoxia, this metabolic adaptation might contribute significantly to the enhanced aggressiveness observed in the SKOV-3 and PA-1 cell lines.
Interestingly, the implications of these findings extend beyond just ovarian cancer. Other malignant cells have been shown to exploit similar mechanisms to overcome hypoxic conditions, making ACSS2 a potentially universal target. This indicates that therapies designed to inhibit ACSS2 may possess broader applications in oncology, thereby increasing their significance.
Investigating the interaction between ACSS2 and other signaling pathways present in the tumor microenvironment revealed even more complexity. The data suggest that ACSS2 does not work in isolation; rather, it may cooperate with various oncogenic pathways to promote tumorigenesis. Understanding these interactions could offer deeper insights into the multifaceted nature of cancer biology.
The potential of ACSS2 as a therapeutic target sets the stage for future research designed to explore inhibitors that can disrupt its function. With ongoing advancements in drug development, the possibility of targeting metabolic pathways within cancer cells is becoming increasingly feasible. The development of such inhibitors could hold transformative potential for patients suffering from advanced ovarian cancer, offering a new lifeline where conventional therapies have failed.
In addition to the fundamental science, the socio-economic implications of these findings cannot be overlooked. Ovarian cancer, often diagnosed at advanced stages, presents a significant challenge to treatment paradigms. With the advent of personalized medicine, identifying critical metabolic pathways like that of ACSS2 will become essential in tailoring treatment strategies. Improving outcomes for patients hinges on our ability to dissect these intricate biological mechanisms.
Furthermore, the study serves as a clarion call for continued funding and research into understudied areas of cancer biology, particularly as they relate to tumor metabolism. It also highlights the need for interdisciplinary approaches that meld oncology with biochemistry, genetics, and molecular biology to fully understand cancer’s adaptive capacities.
ACSS2’s exploration can thus be seen as part of a larger quest to unmask the intricacies of cancer metabolism, which continues to be a crucial frontier in the fight against cancer. The potential developments stemming from this research could not only enhance our arsenal against ovarian cancer but also contribute to broader efforts aimed at addressing malignancies through metabolic interventions.
In summary, Mroweh et al.’s research adds an important chapter to our understanding of cancer biology, providing new lenses through which to view the metabolic derangements associated with malignancies. The role of ACSS2 in promoting proliferation and invasion of ovarian cancer cells under hypoxia could lead to innovative therapeutic strategies that redefine treatment protocols for patients facing this formidable disease.
The integration of these findings into clinical contexts will undoubtedly require rigorous validation and extensive trials, yet the promise they hold is undeniable. As research continues to unveil the complexities of cancer, the marriage of metabolic insights with therapeutic development offers hope for more effective, personalized cancer treatments. As we look towards the future, the potential to disrupt the metabolic adaptations of cancer holds the key to unlocking a new era in cancer therapy.
Subject of Research: Ovarian cancer proliferation and invasiveness mechanisms under hypoxia related to ACSS2 expression.
Article Title: ACSS2 promotes proliferation and invasiveness of SKOV-3 and PA-1 ovarian cancer cell lines under hypoxia.
Article References:
Mroweh, O., Karam, L., Hammoud, R. et al. ACSS2 promotes proliferation and invasiveness of SKOV-3 and PA-1 ovarian cancer cell lines under hypoxia.
J Ovarian Res 18, 232 (2025). https://doi.org/10.1186/s13048-025-01815-y
Image Credits: AI Generated
DOI: 10.1186/s13048-025-01815-y
Keywords: ACSS2, ovarian cancer, hypoxia, metabolic adaptation, therapeutic target.
Tags: ACSS2 expression in cancerACSS2 role in ovarian canceradaptations to low oxygen levelscancer cell metabolic reprogrammingcancer proliferation in hypoxic conditionscellular metabolism in hypoxiahypoxia in tumor microenvironmentinvasiveness of cancer cellsovarian cancer cell growth mechanismsresistance to conventional cancer therapiesSKOV-3 and PA-1 cell linestherapeutic targets for ovarian cancer